Achromatopsia, Color Vision, and Cortex Charles A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ametropia and Emmetropization in CNGB3 Achromatopsia
Retina Ametropia and Emmetropization in CNGB3 Achromatopsia Mette Kjøbæk Gundestrup Andersen1 and Line Kessel1,2 1Department of Ophthalmology, Copenhagen University Hospital, Rigshospitalet-Glostrup, Glostrup, Denmark 2Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark Correspondence: Mette K.G. PURPOSE. Emmetropization is the process of adjusting ocular growth to the focal plane Andersen, Department of in order to achieve a clear image. Chromatic light may be involved as a cue to guide Ophthalmology, Copenhagen this process. Achromats are color blind and lack normal cone function; they are often University Hospital, described as being hyperopic, indicating a failure to emmetropize. We aim to describe Rigshospitalet-Glostrup, Valdemar the refraction and refractive development in a population of genetically characterized Hansens Vej 1-23, 2600 Glostrup, Denmark; achromats. [email protected]. METHODS. Refractive error data were collected retrospectively from 28 medical records CNGB3 Received: August 23, 2020 of c.1148delC homozygous achromats. The distribution of spherical equivalent Accepted: January 18, 2021 refractive error (SER) and spherical error was analyzed in adults. The refractive develop- Published: February 9, 2021 ment in children was analyzed by documenting astigmatic refractive error and calculating Citation: Andersen MKG, Kessel L. median SER in 1-year age groups and by analyzing the individual development when Ametropia and emmetropization in possible. CNGB3 Invest achromatopsia. RESULTS. The distribution of SER and spherical error resembled a Gaussian distribution, Ophthalmol Vis Sci. 2021;62(2):10. indicating that emmetropization was disturbed in achromats, but we found indication of https://doi.org/10.1167/iovs.62.2.10 some decrease in SER during the first years of childhood. -

Color Blindness LEVELED BOOK • W a Reading A–Z Level W Leveled Book Word Count: 1,349 Color Blindness Connections Writing Choose Two Forms of Color Blindness
Color Blindness LEVELED BOOK • W A Reading A–Z Level W Leveled Book Word Count: 1,349 Color Blindness Connections Writing Choose two forms of color blindness. Write a report that compares the two conditions and their effects on a person’s life. Math Research the statistics about the number of people with the various forms of color blindness in your country. Organize your results in a pie chart. • W Q •T Written by Cheryl Reifsnyder Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com Words to Know Color ancestry molecules complementary photopigments cone cells prism Blindness defects retina genetic disorder rod cells hereditary wavelengths Photo Credits: Front cover, Back cover: © pretoperola/123RF; title page (Both): © shironosov/ iStock/Thinkstock; page 3: © Véronique Burger/Science Source; page 4 (Both): © Michael Shake/Dreamstime.com; page 5: © Designua/Dreamstime.com; page 6 (top): © Gunilla Elam/Science Source; page 6 (Bottom): © Steve Gschmeissner/Science Source; page 7 (Both): © Ted Kinsman/ Science Source; page 8 (left, Both): © anatchant/iStock/Thinkstock; page 8 (center, Both): © Nadezhda Bolotina/Hemera/Thinkstock; page 8 (right, Both): © iremphotography/iStock/Thinkstock; page 9 (all): © Popartic/iStock Editorial/Thinkstock; page 10: © RVN/Alamy Stock Photo; page 12: © Alexander Kaludov/123RF; page 13: © EnChroma; page 14: © Neitz LaBoratory; page 15: © Phanie/Alamy Stock Photo Written by Cheryl Reifsnyder www.readinga-z.com Focus Question Color Blindness Level W Leveled Book Correlation © Learning A–Z LEVEL W Written By Cheryl Reifsnyder What causes color blindness, and how Fountas & Pinnell S can it affect a person’s life? All rights reserved. -
RETINAL DISORDERS Eye63 (1)
RETINAL DISORDERS Eye63 (1) Retinal Disorders Last updated: May 9, 2019 CENTRAL RETINAL ARTERY OCCLUSION (CRAO) ............................................................................... 1 Pathophysiology & Ophthalmoscopy ............................................................................................... 1 Etiology ............................................................................................................................................ 2 Clinical Features ............................................................................................................................... 2 Diagnosis .......................................................................................................................................... 2 Treatment ......................................................................................................................................... 2 BRANCH RETINAL ARTERY OCCLUSION ................................................................................................ 3 CENTRAL RETINAL VEIN OCCLUSION (CRVO) ..................................................................................... 3 Pathophysiology & Etiology ............................................................................................................ 3 Clinical Features ............................................................................................................................... 3 Diagnosis ......................................................................................................................................... -
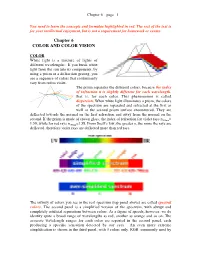
Chapter 6 COLOR and COLOR VISION
Chapter 6 – page 1 You need to learn the concepts and formulae highlighted in red. The rest of the text is for your intellectual enjoyment, but is not a requirement for homework or exams. Chapter 6 COLOR AND COLOR VISION COLOR White light is a mixture of lights of different wavelengths. If you break white light from the sun into its components, by using a prism or a diffraction grating, you see a sequence of colors that continuously vary from red to violet. The prism separates the different colors, because the index of refraction n is slightly different for each wavelength, that is, for each color. This phenomenon is called dispersion. When white light illuminates a prism, the colors of the spectrum are separated and refracted at the first as well as the second prism surface encountered. They are deflected towards the normal on the first refraction and away from the normal on the second. If the prism is made of crown glass, the index of refraction for violet rays n400nm= 1.59, while for red rays n700nm=1.58. From Snell’s law, the greater n, the more the rays are deflected, therefore violet rays are deflected more than red rays. The infinity of colors you see in the real spectrum (top panel above) are called spectral colors. The second panel is a simplified version of the spectrum, with abrupt and completely artificial separations between colors. As a figure of speech, however, we do identify quite a broad range of wavelengths as red, another as orange and so on. -

Cortical Mechanisms of Colour Vision
REVIEWS CORTICAL MECHANISMS OF COLOUR VISION Karl R. Gegenfurtner The perception of colour is a central component of primate vision. Colour facilitates object perception and recognition, and has an important role in scene segmentation and visual memory. Moreover, it provides an aesthetic component to visual experiences that is fundamental to our perception of the world. Despite the long history of colour vision studies, much has still to be learned about the physiological basis of colour perception. Recent advances in our understanding of the early processing in the retina and thalamus have enabled us to take a fresh look at cortical processing of colour. These studies are beginning to indicate that colour is processed not in isolation, but together with information about luminance and visual form, by the same neural circuits, to achieve a unitary and robust representation of the visual world. SENSORY SYSTEMS It has been known since the nineteenth century that In the retinal ganglion cells, three channels convey there are three types of photoreceptor for daylight information from the eye to the brain7,8. In the L + M or vision in the human eye1,2. Since then, the shapes of the luminance channel, the signals from L- and M-cones spectral absorption functions of the different cones are added to compute the intensity of a stimulus. In the have been determined with increasing precision in L – M colour-opponent channel, the signals from L- and psychophysical experiments and verified using various M-cones are subtracted from each other to compute electrophysiological methods3. On the molecular the red–green component of a stimulus. -

Rethinking Color Cameras
Rethinking Color Cameras Ayan Chakrabarti William T. Freeman Todd Zickler Harvard University Massachusetts Institute of Technology Harvard University Cambridge, MA Cambridge, MA Cambridge, MA [email protected] [email protected] [email protected] Abstract Digital color cameras make sub-sampled measurements of color at alternating pixel locations, and then “demo- saick” these measurements to create full color images by up-sampling. This allows traditional cameras with re- stricted processing hardware to produce color images from a single shot, but it requires blocking a majority of the in- cident light and is prone to aliasing artifacts. In this paper, we introduce a computational approach to color photogra- phy, where the sampling pattern and reconstruction process are co-designed to enhance sharpness and photographic speed. The pattern is made predominantly panchromatic, thus avoiding excessive loss of light and aliasing of high spatial-frequency intensity variations. Color is sampled at a very sparse set of locations and then propagated through- out the image with guidance from the un-aliased luminance channel. Experimental results show that this approach often leads to significant reductions in noise and aliasing arti- facts, especially in low-light conditions. Figure 1. A computational color camera. Top: Most digital color cameras use the Bayer pattern (or something like it) to sub-sample 1. Introduction color alternatingly; and then they demosaick these samples to create full-color images by up-sampling. Bottom: We propose The standard practice for one-shot digital color photog- an alternative that samples color very sparsely and is otherwise raphy is to include a color filter array in front of the sensor panchromatic. -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

Spatial Filtering, Color Constancy, and the Color-Changing Dress Department of Psychology, American University, Erica L
Journal of Vision (2017) 17(3):7, 1–20 1 Spatial filtering, color constancy, and the color-changing dress Department of Psychology, American University, Erica L. Dixon Washington, DC, USA Department of Psychology and Department of Computer Arthur G. Shapiro Science, American University, Washington, DC, USA The color-changing dress is a 2015 Internet phenomenon divide among responders (as well as disagreement from in which the colors in a picture of a dress are reported as widely followed celebrity commentators) fueled a rapid blue-black by some observers and white-gold by others. spread of the photo across many online news outlets, The standard explanation is that observers make and the topic trended worldwide on Twitter under the different inferences about the lighting (is the dress in hashtag #theDress. The huge debate on the Internet shadow or bright yellow light?); based on these also sparked debate in the vision science community inferences, observers make a best guess about the about the implications of the stimulus with regard to reflectance of the dress. The assumption underlying this individual differences in color perception, which in turn explanation is that reflectance is the key to color led to a special issue of the Journal of Vision, for which constancy because reflectance alone remains invariant this article is written. under changes in lighting conditions. Here, we The predominant explanations in both scientific demonstrate an alternative type of invariance across journals (Gegenfurtner, Bloj, & Toscani, 2015; Lafer- illumination conditions: An object that appears to vary in Sousa, Hermann, & Conway, 2015; Winkler, Spill- color under blue, white, or yellow illumination does not change color in the high spatial frequency region. -

OCULAR GENE THERAPY TRIALS ADVANCE Results of the First Phase 3 Trial Were Announced
OCULAR GENE THERAPY TRIALS ADVANCE Results of the first phase 3 trial were announced. BY ARON SHAPIRO INNOVATIONS IN RETINA INNOVATIONS “We used to think that our fate was in our AAV FACILITATES GENE DELIVERY stars, but now we know that, in large measure, The nonpathogenic adeno-associated virus (AAV) has our fate is in our genes.” to date been a safe and effective vector for gene delivery. –James Watson1 The recombinant AAV (rAAV) vector has demonstrated increased specificity and efficiency in ocular AAV-mediated Genetic alterations are known to be respon- gene therapy interventions. Recombinant AAV2 (rAAV2) sible for numerous diseases, and so it follows vectors used for gene therapy are derived from the wild-type logically that the best cures for these diseases virus by deleting the entire viral coding region and replac- might lie in correcting genetic anomalies by the process of ing it with the reporter or therapeutic transgene. Combined gene therapy. This approach involves the introduction of AAV serotypes have also been developed. This diversity genes into existing cells in attempts to prevent or cure a of serotypes may lead to more specific and more efficient wide range of diseases previously thought to be incurable.1 transduction. However, successful and efficient transfection Posterior segment disorders are challenging to treat, and of particular cell types in the eye still depends on other fac- current therapies have numerous shortcomings. Many are tors, such as the AAV titer, the site of injection, the amount invasive, run the risk of complications, offer only short-term of passenger DNA, and the specific gene promoters where relief from symptoms, or are unable to directly treat vision transcription initiation takes place.2 loss. -

Color Constancy and Contextual Effects on Color Appearance
Chapter 6 Color Constancy and Contextual Effects on Color Appearance Maria Olkkonen and Vebjørn Ekroll Abstract Color is a useful cue to object properties such as object identity and state (e.g., edibility), and color information supports important communicative functions. Although the perceived color of objects is related to their physical surface properties, this relationship is not straightforward. The ambiguity in perceived color arises because the light entering the eyes contains information about both surface reflectance and prevailing illumination. The challenge of color constancy is to estimate surface reflectance from this mixed signal. In addition to illumination, the spatial context of an object may also affect its color appearance. In this chapter, we discuss how viewing context affects color percepts. We highlight some important results from previous research, and move on to discuss what could help us make further progress in the field. Some promising avenues for future research include using individual differences to help in theory development, and integrating more naturalistic scenes and tasks along with model comparison into color constancy and color appearance research. Keywords Color perception • Color constancy • Color appearance • Context • Psychophysics • Individual differences 6.1 Introduction Color is a useful cue to object properties such as object identity and state (e.g., edibility), and color information supports important communicative functions [1]. Although the perceived color of objects is related to their physical surface properties, M. Olkkonen, M.A. (Psych), Dr. rer. nat. (*) Department of Psychology, Science Laboratories, Durham University, South Road, Durham DH1 3LE, UK Institute of Behavioural Sciences, University of Helsinki, Siltavuorenpenger 1A, 00014 Helsinki, Finland e-mail: [email protected]; maria.olkkonen@helsinki.fi V. -

Colour Vision Deficiency
Eye (2010) 24, 747–755 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye Colour vision MP Simunovic REVIEW deficiency Abstract effective "treatment" of colour vision deficiency: whilst it has been suggested that tinted lenses Colour vision deficiency is one of the could offer a means of enabling those with commonest disorders of vision and can be colour vision deficiency to make spectral divided into congenital and acquired forms. discriminations that would normally elude Congenital colour vision deficiency affects as them, clinical trials of such lenses have been many as 8% of males and 0.5% of femalesFthe largely disappointing. Recent developments in difference in prevalence reflects the fact that molecular genetics have enabled us to not only the commonest forms of congenital colour understand more completely the genetic basis of vision deficiency are inherited in an X-linked colour vision deficiency, they have opened the recessive manner. Until relatively recently, our possibility of gene therapy. The application of understanding of the pathophysiological basis gene therapy to animal models of colour vision of colour vision deficiency largely rested on deficiency has shown dramatic results; behavioural data; however, modern molecular furthermore, it has provided interesting insights genetic techniques have helped to elucidate its into the plasticity of the visual system with mechanisms. respect to extracting information about the The current management of congenital spectral composition of the visual scene. colour vision deficiency lies chiefly in appropriate counselling (including career counselling). Although visual aids may Materials and methods be of benefit to those with colour vision deficiency when performing certain tasks, the This article was prepared by performing a evidence suggests that they do not enable primary search of Pubmed for articles on wearers to obtain normal colour ‘colo(u)r vision deficiency’ and ‘colo(u)r discrimination. -

1 Human Color Vision
CAMC01 9/30/04 3:13 PM Page 1 1 Human Color Vision Color appearance models aim to extend basic colorimetry to the level of speci- fying the perceived color of stimuli in a wide variety of viewing conditions. To fully appreciate the formulation, implementation, and application of color appearance models, several fundamental topics in color science must first be understood. These are the topics of the first few chapters of this book. Since color appearance represents several of the dimensions of our visual experience, any system designed to predict correlates to these experiences must be based, to some degree, on the form and function of the human visual system. All of the color appearance models described in this book are derived with human visual function in mind. It becomes much simpler to understand the formulations of the various models if the basic anatomy, physiology, and performance of the visual system is understood. Thus, this book begins with a treatment of the human visual system. As necessitated by the limited scope available in a single chapter, this treatment of the visual system is an overview of the topics most important for an appreciation of color appearance modeling. The field of vision science is immense and fascinating. Readers are encouraged to explore the liter- ature and the many useful texts on human vision in order to gain further insight and details. Of particular note are the review paper on the mechan- isms of color vision by Lennie and D’Zmura (1988), the text on human color vision by Kaiser and Boynton (1996), the more general text on the founda- tions of vision by Wandell (1995), the comprehensive treatment by Palmer (1999), and edited collections on color vision by Backhaus et al.