Species-Level Microbiome Composition of Activated Sludge - Introducing the Midas 3 2 Ecosystem-Specific Reference Database and Taxonomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metaproteogenomic Insights Beyond Bacterial Response to Naphthalene
ORIGINAL ARTICLE ISME Journal – Original article Metaproteogenomic insights beyond bacterial response to 5 naphthalene exposure and bio-stimulation María-Eugenia Guazzaroni, Florian-Alexander Herbst, Iván Lores, Javier Tamames, Ana Isabel Peláez, Nieves López-Cortés, María Alcaide, Mercedes V. del Pozo, José María Vieites, Martin von Bergen, José Luis R. Gallego, Rafael Bargiela, Arantxa López-López, Dietmar H. Pieper, Ramón Rosselló-Móra, Jesús Sánchez, Jana Seifert and Manuel Ferrer 10 Supporting Online Material includes Text (Supporting Materials and Methods) Tables S1 to S9 Figures S1 to S7 1 SUPPORTING TEXT Supporting Materials and Methods Soil characterisation Soil pH was measured in a suspension of soil and water (1:2.5) with a glass electrode, and 5 electrical conductivity was measured in the same extract (diluted 1:5). Primary soil characteristics were determined using standard techniques, such as dichromate oxidation (organic matter content), the Kjeldahl method (nitrogen content), the Olsen method (phosphorus content) and a Bernard calcimeter (carbonate content). The Bouyoucos Densimetry method was used to establish textural data. Exchangeable cations (Ca, Mg, K and 10 Na) extracted with 1 M NH 4Cl and exchangeable aluminium extracted with 1 M KCl were determined using atomic absorption/emission spectrophotometry with an AA200 PerkinElmer analyser. The effective cation exchange capacity (ECEC) was calculated as the sum of the values of the last two measurements (sum of the exchangeable cations and the exchangeable Al). Analyses were performed immediately after sampling. 15 Hydrocarbon analysis Extraction (5 g of sample N and Nbs) was performed with dichloromethane:acetone (1:1) using a Soxtherm extraction apparatus (Gerhardt GmbH & Co. -

Supplementary Information for Microbial Electrochemical Systems Outperform Fixed-Bed Biofilters for Cleaning-Up Urban Wastewater
Electronic Supplementary Material (ESI) for Environmental Science: Water Research & Technology. This journal is © The Royal Society of Chemistry 2016 Supplementary information for Microbial Electrochemical Systems outperform fixed-bed biofilters for cleaning-up urban wastewater AUTHORS: Arantxa Aguirre-Sierraa, Tristano Bacchetti De Gregorisb, Antonio Berná, Juan José Salasc, Carlos Aragónc, Abraham Esteve-Núñezab* Fig.1S Total nitrogen (A), ammonia (B) and nitrate (C) influent and effluent average values of the coke and the gravel biofilters. Error bars represent 95% confidence interval. Fig. 2S Influent and effluent COD (A) and BOD5 (B) average values of the hybrid biofilter and the hybrid polarized biofilter. Error bars represent 95% confidence interval. Fig. 3S Redox potential measured in the coke and the gravel biofilters Fig. 4S Rarefaction curves calculated for each sample based on the OTU computations. Fig. 5S Correspondence analysis biplot of classes’ distribution from pyrosequencing analysis. Fig. 6S. Relative abundance of classes of the category ‘other’ at class level. Table 1S Influent pre-treated wastewater and effluents characteristics. Averages ± SD HRT (d) 4.0 3.4 1.7 0.8 0.5 Influent COD (mg L-1) 246 ± 114 330 ± 107 457 ± 92 318 ± 143 393 ± 101 -1 BOD5 (mg L ) 136 ± 86 235 ± 36 268 ± 81 176 ± 127 213 ± 112 TN (mg L-1) 45.0 ± 17.4 60.6 ± 7.5 57.7 ± 3.9 43.7 ± 16.5 54.8 ± 10.1 -1 NH4-N (mg L ) 32.7 ± 18.7 51.6 ± 6.5 49.0 ± 2.3 36.6 ± 15.9 47.0 ± 8.8 -1 NO3-N (mg L ) 2.3 ± 3.6 1.0 ± 1.6 0.8 ± 0.6 1.5 ± 2.0 0.9 ± 0.6 TP (mg -

Core Bacterial Taxon from Municipal Wastewater Treatment Plants
ENVIRONMENTAL MICROBIOLOGY crossm Casimicrobium huifangae gen. nov., sp. nov., a Ubiquitous “Most-Wanted” Core Bacterial Taxon from Municipal Wastewater Treatment Plants Yang Song,a,b,c,d,g Cheng-Ying Jiang,a,b,c,d Zong-Lin Liang,a,b,c,g Bao-Jun Wang,a Yong Jiang,e Ye Yin,f Hai-Zhen Zhu,a,b,c,g Ya-Ling Qin,a,b,c,g Rui-Xue Cheng,a Zhi-Pei Liu,a,b,c,d Yao Liu,e Tao Jin,f Philippe F.-X. Corvini,h Korneel Rabaey,i Downloaded from Ai-Jie Wang,a,d,g Shuang-Jiang Liua,b,c,d,g aKey Laboratory of Environmental Biotechnology at Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, China bState Key Laboratory of Microbial Resources at Institute of Microbiology, Chinese Academy of Sciences, Beijing, China cEnvironmental Microbiology Research Center at Institute of Microbiology, Chinese Academy of Sciences, Beijing, China dRCEES-IMCAS-UCAS Joint-Lab of Microbial Technology for Environmental Science, Beijing, China eBeijing Drainage Group Co., Ltd., Beijing, China f BGI-Qingdao, Qingdao, China http://aem.asm.org/ gUniversity of Chinese Academy of Sciences, Beijing, China hUniversity of Applied Sciences and Arts Northwestern Switzerland, Muttenz, Switzerland iCenter for Microbial Ecology and Technology (CMET), Ghent University, Ghent, Belgium Yang Song and Cheng-Ying Jiang contributed equally to this work. Author order was determined by drawing straws. ABSTRACT Microorganisms in wastewater treatment plants (WWTPs) play a key role in the removal of pollutants from municipal and industrial wastewaters. A recent study estimated that activated sludge from global municipal WWTPs har- on June 19, 2020 by guest bors 1 ϫ 109 to 2 ϫ 109 microbial species, the majority of which have not yet been cultivated, and 28 core taxa were identified as “most-wanted” ones (L. -

“Candidatus Propionivibrio Aalborgensis”: a Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants
fmicb-07-01033 June 30, 2016 Time: 16:42 # 1 ORIGINAL RESEARCH published: 04 July 2016 doi: 10.3389/fmicb.2016.01033 “Candidatus Propionivibrio aalborgensis”: A Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants Mads Albertsen1†, Simon J. McIlroy1†, Mikkel Stokholm-Bjerregaard1,2, Søren M. Karst1 and Per H. Nielsen1* 1 Center for Microbial Communities, Department of Chemistry and Bioscience, Aalborg University, Aalborg, Denmark, 2 Edited by: Krüger A/S, Aalborg, Denmark Gene W. Tyson, University of Queensland, Australia Enhanced biological phosphorus removal (EBPR) is widely used to remove phosphorus Reviewed by: from wastewater. The process relies on polyphosphate accumulating organisms (PAOs) Katherine McMahon, University of Wisconsin–Madison, that are able to take up phosphorus in excess of what is needed for growth, USA whereby phosphorus can be removed from the wastewater by wasting the biomass. Connor Tobias Skennerton, California Institute of Technology, USA However, glycogen accumulating organisms (GAOs) may reduce the EBPR efficiency *Correspondence: as they compete for substrates with PAOs, but do not store excessive amounts of Per H. Nielsen polyphosphate. PAOs and GAOs are thought to be phylogenetically unrelated, with [email protected] the model PAO being the betaproteobacterial “Candidatus Accumulibacter phosphatis” www.bio.aau.dk (Accumulibacter) and the model GAO being the gammaproteobacterial “Candidatus †These authors have contributed equally to this work. Competibacter phosphatis”. Here, we report the discovery of a GAO from the genus Propionivibrio, which is closely related to Accumulibacter. Propionivibrio sp. Specialty section: This article was submitted to are targeted by the canonical fluorescence in situ hybridization probes used to target Microbial Physiology and Metabolism, Accumulibacter (PAOmix), but do not store excessive amounts of polyphosphate in a section of the journal situ. -

“Candidatus Propionivibrio Aalborgensis”: a Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants
Aalborg Universitet “Candidatus Propionivibrio aalborgensis” A Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants Albertsen, Mads; McIlroy, Simon Jon; Stokholm-Bjerregaard, Mikkel; Karst, Søren Michael; Nielsen, Per Halkjær Published in: Frontiers in Microbiology DOI (link to publication from Publisher): 10.3389/fmicb.2016.01033 Creative Commons License CC BY 4.0 Publication date: 2016 Document Version Publisher's PDF, also known as Version of record Link to publication from Aalborg University Citation for published version (APA): Albertsen, M., McIlroy, S. J., Stokholm-Bjerregaard, M., Karst, S. M., & Nielsen, P. H. (2016). “Candidatus Propionivibrio aalborgensis”: A Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants. Frontiers in Microbiology, 7, [1033]. https://doi.org/10.3389/fmicb.2016.01033 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. ? Users may download and print one copy of any publication from the public portal for the purpose of private study or research. ? You may not further distribute the material or use it for any profit-making activity or commercial gain ? You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us at [email protected] providing details, and we will remove access to the work immediately and investigate your claim. fmicb-07-01033 June 30, 2016 Time: 16:42 # 1 ORIGINAL RESEARCH published: 04 July 2016 doi: 10.3389/fmicb.2016.01033 “Candidatus Propionivibrio aalborgensis”: A Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants Mads Albertsen1†, Simon J. -

Albertsen, Mads, Mcilroy, Simon J., Stokholm-Bjerregaard, Mikkel, Karst, Søren M., & Nielsen, Per H
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Albertsen, Mads, McIlroy, Simon J., Stokholm-Bjerregaard, Mikkel, Karst, Søren M., & Nielsen, Per H. (2016) "Candidatus Propionivibrio aalborgensis":A novel glycogen accumulating organism abundant in full-scale enhanced biological phosphorus removal plants. Frontiers in Microbiology, 7, Article number: 1033. This file was downloaded from: https://eprints.qut.edu.au/205764/ c The Author(s) 2016 This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] License: Creative Commons: Attribution 4.0 Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.3389/fmicb.2016.01033 fmicb-07-01033 June 30, 2016 Time: 16:42 # 1 ORIGINAL RESEARCH published: 04 July 2016 doi: 10.3389/fmicb.2016.01033 “Candidatus Propionivibrio aalborgensis”: A Novel Glycogen Accumulating Organism Abundant in Full-Scale Enhanced Biological Phosphorus Removal Plants Mads Albertsen1†, Simon J. -

Abstract Tracing Hydrocarbon
ABSTRACT TRACING HYDROCARBON CONTAMINATION THROUGH HYPERALKALINE ENVIRONMENTS IN THE CALUMET REGION OF SOUTHEASTERN CHICAGO Kathryn Quesnell, MS Department of Geology and Environmental Geosciences Northern Illinois University, 2016 Melissa Lenczewski, Director The Calumet region of Southeastern Chicago was once known for industrialization, which left pollution as its legacy. Disposal of slag and other industrial wastes occurred in nearby wetlands in attempt to create areas suitable for future development. The waste creates an unpredictable, heterogeneous geology and a unique hyperalkaline environment. Upgradient to the field site is a former coking facility, where coke, creosote, and coal weather openly on the ground. Hydrocarbons weather into characteristic polycyclic aromatic hydrocarbons (PAHs), which can be used to create a fingerprint and correlate them to their original parent compound. This investigation identified PAHs present in the nearby surface and groundwaters through use of gas chromatography/mass spectrometry (GC/MS), as well as investigated the relationship between the alkaline environment and the organic contamination. PAH ratio analysis suggests that the organic contamination is not mobile in the groundwater, and instead originated from the air. 16S rDNA profiling suggests that some microbial communities are influenced more by pH, and some are influenced more by the hydrocarbon pollution. BIOLOG Ecoplates revealed that most communities have the ability to metabolize ring structures similar to the shape of PAHs. Analysis with bioinformatics using PICRUSt demonstrates that each community has microbes thought to be capable of hydrocarbon utilization. The field site, as well as nearby areas, are targets for habitat remediation and recreational development. In order for these remediation efforts to be successful, it is vital to understand the geochemistry, weathering, microbiology, and distribution of known contaminants. -

Isolation and Characterization of Bacteria in a Toluene-Producing Enrichment Culture Derived from Contaminated Groundwater at a Louisiana Superfund Site
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School August 2020 Isolation and Characterization of Bacteria in a Toluene-Producing Enrichment Culture Derived from Contaminated Groundwater at a Louisiana Superfund Site Madison Mikes Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Civil and Environmental Engineering Commons, and the Microbiology Commons Recommended Citation Mikes, Madison, "Isolation and Characterization of Bacteria in a Toluene-Producing Enrichment Culture Derived from Contaminated Groundwater at a Louisiana Superfund Site" (2020). LSU Master's Theses. 5206. https://digitalcommons.lsu.edu/gradschool_theses/5206 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ISOLATION AND CHARACTERIZATION OF BACTERIA IN A TOLUENE- PRODUCING ENRICHMENT CULTURE DERIVED FROM CONTAMINATED GROUNDWATER AT A LOUISIANA SUPERFUND SITE A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agriculture and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Civil and Environmental Engineering by Madison Colleen Mikes B.S., Louisiana State University, 2018 December 2020 1 ACKNOWLEDGEMENTS I would like to take the time to thank all of those who have supported and assisted me during my graduate program. First and foremost, I would like to thank Dr. Bill Moe for all of the time he has spent teaching me and mentoring me through my thesis work. -

Composition of Bacterial and Archaeal Communities in Freshwater Sediments with Different Contamination Levels (Lake Geneva, Switzerland)
water research 45 (2011) 1213e1228 Available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/watres Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland) Laurence Haller a, Mauro Tonolla b,d, Jakob Zopfi c, Raffaele Peduzzi b, Walter Wildi a, John Pote´ a,* a University of Geneva, Institute F.A. Forel, 10 route de Suisse, CP 416, CH-1290 Versoix, Switzerland b Microbial Ecology, Microbiology Unit, Plant Biology Department, University of Geneva, 30, Quai Ernest-Ansermet, CH-1211 Geneva, Switzerland c University of Lausanne, Institute of Geology and Paleontology, Laboratory of Biogeosciences, CH-1015 Lausanne, Switzerland d Cantonal Institute of Microbiology, Via Mirasole 22A, CH-6500 Bellinzona, Switzerland article info abstract Article history: The aim of this study was to compare the composition of bacterial and archaeal Received 31 August 2010 communities in contaminated sediments (Vidy Bay) with uncontaminated sediments Received in revised form (Ouchy area) of Lake Geneva using 16S rRNA clone libraries. Sediments of both sites were 8 November 2010 analysed for physicochemical characteristics including porewater composition, organic Accepted 14 November 2010 carbon, and heavy metals. Results show high concentrations of contaminants in sediments Available online 20 November 2010 from Vidy. Particularly, high contents of fresh organic matter and nutrients led to intense mineralisation, which was dominated by sulphate-reduction and methanogenesis. The Keywords: bacterial diversity in Vidy sediments was significantly different from the communities in Lake Geneva the uncontaminated sediments. Phylogenetic analysis revealed a large proportion of Sediment pollution Betaproteobacteria clones in Vidy sediments related to Dechloromonas sp., a group of dech- Heavy metal lorinating and contaminant degrading bacteria. -
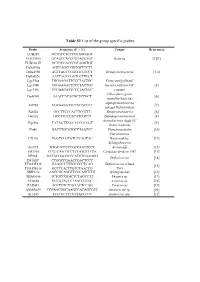
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

Towards a Metranscriptomic Comparison of Two Alpine Soils Under Contrasted Snow Cover
THÈSE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITÉ DE GRENOBLE Spécialité : Méthodes et algorithmes en Biologie Présentée par Tarfa Mustafa Thèse dirigée par Roberto A. Geremia et codirigée par Jean Martins Préparée au sein du Laboratoire d’Ecologie Alpine Dans l'École Doctorale Ingénierie de la santé, la cognition et l’environnement Towards a metranscriptomic comparison of two alpine soils under contrasted snow cover Thèse soutenue publiquement le 28 Septembre 2011, devant le jury composé de : Rolland Marmeisse: CR CNRS Ecologie Microbienne Lyon ( rapporteur ) Denis Faure: DR CNRS, Institut des Sciences du Végétal, Gif-sur-Yvette ( rapporteur ) Lionel Ranjard: DR INRA, Genosol, Dijon ( examinateur ) Patrick Ravanel: Prof, UJF, LECA, Grenoble ( examinateur ) Jean Martins: CR CNRS, LTHE, Grenoble, ( co-directeur de thèse ) Roberto A. Geremia: DR CNRS- LECA, Grenoble, ( directeur de thèse ) Table of content Remerciements Présentation de la démarche Chapter I - literature Review 1 2.1 Soil environment in general, who is there? 1 2.1.1 Soil environment, formation and characteristics. 1 2.1.2 Soil biodiversity: definition and concept. 1 2.1.3 Microbial diversity estimations methods. 4 2.1.4 Eukaryotes as major component of soil environment. 7 2.1.4.1 Eukaryotes evolution history. 7 2.1.4.2 Fungal phylogeny. 8 2.2 Soil ecosystem, who is there, and what they are doing? 13 2.2.1 What is an ecosystem? 13 2.2.2 The soil ecosystem, concept, definition and key elements. 14 2.2.2.1 Major factors influencing soil ecosystem. 15 2.2.2.2 Above- and belowground interaction effects on ecosystem functions. -

Suppl. Figure 1 (.Pdf)
Supplementary web Figure 1. 16S rRNA-based phylogenetic trees showing the affiliation of all cultured and uncultured members of the nine “Rhodocyclales” lineages. The consensus tree is based on maximum-likelihood analysis (AxML) of full- length sequences (>1,300 nucleotides) performed with a 50% conservation filter for the “Betaproteobacteria”. Named type species are indicated by boldface type. Bar indicates 10% estimated sequence divergence. Polytomic nodes connect branches for which a relative order could not be determined unambiguously by applying neighbor- joining, maximum-parsimony, and maximum-likelihood treeing methods. Numbers at branches indicate parsimony bootstrap values in percent. Branches without numbers had bootstrap values of less than 75%. The minimum 16S rRNA sequence similarity for each “Rhodocyclales” lineage is shown. P+ sludge clone SBR1021, AF204250 P+ sludge clone GC152, AF204242 Kraftisried wwtp clone KRA42, AY689087 Kraftisried wwtp clone S28, AF072922 Kraftisried wwtp clone A13, AF072927 Kraftisried wwtp clone H23, AF072926 Kraftisried wwtp clone S40, AF234757 Sterolibacterium lineage 100 Kraftisried wwtp clone H12, AF072923 100 Kraftisried wwtp clone H20, AF072920 92.5% 89 rape root clone RRA12, AY687926 100 P+ sludge clone SBR1001, AF204252 P- sludge clone SBR2080, AF204251 P+ sludge clone GC24, AF204243 mine water clone I12, AY187895 100 93 denitrifying cholesterol-degrading bacterium 72Chol, Y09967 Sterolibacterium denitrificans, AJ306683 Kraftisried wwtp clone KRZ64, AY689092 Kraftisried wwtp clone KRZ70,