Towards a Metranscriptomic Comparison of Two Alpine Soils Under Contrasted Snow Cover
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metaproteogenomic Insights Beyond Bacterial Response to Naphthalene
ORIGINAL ARTICLE ISME Journal – Original article Metaproteogenomic insights beyond bacterial response to 5 naphthalene exposure and bio-stimulation María-Eugenia Guazzaroni, Florian-Alexander Herbst, Iván Lores, Javier Tamames, Ana Isabel Peláez, Nieves López-Cortés, María Alcaide, Mercedes V. del Pozo, José María Vieites, Martin von Bergen, José Luis R. Gallego, Rafael Bargiela, Arantxa López-López, Dietmar H. Pieper, Ramón Rosselló-Móra, Jesús Sánchez, Jana Seifert and Manuel Ferrer 10 Supporting Online Material includes Text (Supporting Materials and Methods) Tables S1 to S9 Figures S1 to S7 1 SUPPORTING TEXT Supporting Materials and Methods Soil characterisation Soil pH was measured in a suspension of soil and water (1:2.5) with a glass electrode, and 5 electrical conductivity was measured in the same extract (diluted 1:5). Primary soil characteristics were determined using standard techniques, such as dichromate oxidation (organic matter content), the Kjeldahl method (nitrogen content), the Olsen method (phosphorus content) and a Bernard calcimeter (carbonate content). The Bouyoucos Densimetry method was used to establish textural data. Exchangeable cations (Ca, Mg, K and 10 Na) extracted with 1 M NH 4Cl and exchangeable aluminium extracted with 1 M KCl were determined using atomic absorption/emission spectrophotometry with an AA200 PerkinElmer analyser. The effective cation exchange capacity (ECEC) was calculated as the sum of the values of the last two measurements (sum of the exchangeable cations and the exchangeable Al). Analyses were performed immediately after sampling. 15 Hydrocarbon analysis Extraction (5 g of sample N and Nbs) was performed with dichloromethane:acetone (1:1) using a Soxtherm extraction apparatus (Gerhardt GmbH & Co. -

Chlorate Reduction by an Acetogenic Bacterium, Sporomusa Sp., Isolated from an Underground Gas Storage
Appl Microbiol Biotechnol (2010) 88:595–603 DOI 10.1007/s00253-010-2788-8 ENVIRONMENTAL BIOTECHNOLOGY (Per)chlorate reduction by an acetogenic bacterium, Sporomusa sp., isolated from an underground gas storage Melike Balk & Farrakh Mehboob & Antonie H. van Gelder & W. Irene C. Rijpstra & Jaap S. Sinninghe Damsté & Alfons J. M. Stams Received: 12 March 2010 /Revised: 16 July 2010 /Accepted: 16 July 2010 /Published online: 3 August 2010 # The Author(s) 2010. This article is published with open access at Springerlink.com Abstract A mesophilic bacterium, strain An4, was isolated Keywords Sporomusa sp. Perchlorate . from an underground gas storage reservoir with methanol Underground gas storage as substrate and perchlorate as electron acceptor. Cells were Gram-negative, spore-forming, straight to curved rods, 0.5– 0.8 μm in diameter, and 2–8 μm in length, growing as Introduction single cells or in pairs. The cells grew optimally at 37°C, and the pH optimum was around 7. Strain An4 converted Perchlorate and chlorate are used in a wide range of various alcohols, organic acids, fructose, acetoin, and applications. Chlorate is used as an herbicide or defoliant. H2/CO2 to acetate, usually as the only product. Succinate Perchlorate salts have been manufactured in large quantities was decarboxylated to propionate. The isolate was able to and used as ingredients in solid rocket fuels, highway safety respire with (per)chlorate, nitrate, and CO2. The G+C flares, air bag inflators, fireworks, and matches (Renner content of the DNA was 42.6 mol%. Based on the 16S 1998; Logan 2001; Motzer 2001). Perchlorate is chemically rRNA gene sequence analysis, strain An4 was most closely very stable and has low reactivity even in highly reducing related to Sporomusa ovata (98% similarity). -

Repeated Anaerobic Microbial Redox Cycling of Iron †
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Sept. 2011, p. 6036–6042 Vol. 77, No. 17 0099-2240/11/$12.00 doi:10.1128/AEM.00276-11 Copyright © 2011, American Society for Microbiology. All Rights Reserved. Repeated Anaerobic Microbial Redox Cycling of Ironᰔ† Aaron J. Coby,1‡ Flynn Picardal,2 Evgenya Shelobolina,1 Huifang Xu,1 and Eric E. Roden1* Department of Geoscience, University of Wisconsin, Madison, Wisconsin 53706,1 and School of Public and Environmental Affairs, Indiana University, Bloomington, Indiana 474052 Received 7 February 2011/Accepted 28 June 2011 Some nitrate- and Fe(III)-reducing microorganisms are capable of oxidizing Fe(II) with nitrate as the electron acceptor. This enzymatic pathway may facilitate the development of anaerobic microbial communities that take advantage of the energy available during Fe-N redox oscillations. We examined this phenomenon in synthetic Fe(III) oxide (nanocrystalline goethite) suspensions inoculated with microflora from freshwater river floodplain sediments. Nitrate and acetate were added at alternate intervals in order to induce repeated cycles of microbial Fe(III) reduction and nitrate-dependent Fe(II) oxidation. Addition of nitrate to reduced, acetate- depleted suspensions resulted in rapid Fe(II) oxidation and accumulation of ammonium. High-resolution transmission electron microscopic analysis of material from Fe redox cycling reactors showed amorphous coatings on the goethite nanocrystals that were not observed in reactors operated under strictly nitrate- or Fe(III)-reducing conditions. Microbial communities associated with N and Fe redox metabolism were assessed using a combination of most-probable-number enumerations and 16S rRNA gene analysis. The nitrate- reducing and Fe(III)-reducing cultures were dominated by denitrifying Betaproteobacteria (e.g., Dechloromonas) and Fe(III)-reducing Deltaproteobacteria (Geobacter), respectively; these same taxa were dominant in the Fe cycling cultures. -

Bacterial Community Dynamics During the Early Stages of Biofilm Formation
Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration DOUTERELO, I., SHARPE, Rebecca <http://orcid.org/0000-0002-2783-9215> and BOXALL, J. Available from Sheffield Hallam University Research Archive (SHURA) at: http://shura.shu.ac.uk/9517/ This document is the author deposited version. You are advised to consult the publisher's version if you wish to cite from it. Published version DOUTERELO, I., SHARPE, Rebecca and BOXALL, J. (2014). Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration. Journal of applied microbiology, 117 (1), 286-301. Copyright and re-use policy See http://shura.shu.ac.uk/information.html Sheffield Hallam University Research Archive http://shura.shu.ac.uk Journal of Applied Microbiology ISSN 1364-5072 ORIGINAL ARTICLE Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration I. Douterelo1, R. Sharpe2 and J. Boxall1 1 Pennine Water Group, Department of Civil and Structural Engineering, University of Sheffield, Sheffield, UK 2 School of Civil and Building Engineering, Loughborough University, Loughborough, UK Keywords Abstract 16s rRNA sequencing, bacterial community structure, biofilm development, Aims: To characterize bacterial communities during the early stages of biofilm discolouration, drinking water distribution formation and their role in water discolouration in a fully representative, systems, terminal restriction fragment length chlorinated, experimental drinking water distribution systems (DWDS). polymorphism. Methods and Results: Biofilm development was monitored in an experimental DWDS over 28 days; subsequently the system was disturbed by raising hydraulic Correspondence conditions to simulate pipe burst, cleaning or other system conditions. -

Physiology and Biochemistry of Aromatic Hydrocarbon-Degrading Bacteria That Use Chlorate And/Or Nitrate As Electron Acceptor
Invitation for the public defense of my thesis Physiology and biochemistry of aromatic hydrocarbon-degrading of aromatic and biochemistry Physiology bacteria that use chlorate and/or nitrate as electron acceptor as electron nitrate and/or use chlorate that bacteria Physiology and biochemistry Physiology and biochemistry of aromatic hydrocarbon-degrading of aromatic hydrocarbon- degrading bacteria that bacteria that use chlorate and/or nitrate as electron acceptor use chlorate and/or nitrate as electron acceptor The public defense of my thesis will take place in the Aula of Wageningen University (Generall Faulkesweg 1, Wageningen) on December 18 2013 at 4:00 pm. This defense is followed by a reception in Café Carré (Vijzelstraat 2, Wageningen). Margreet J. Oosterkamp J. Margreet Paranimphs Ton van Gelder ([email protected]) Aura Widjaja Margreet J. Oosterkamp ([email protected]) Marjet Oosterkamp (911 W Springfield Ave Apt 19, Urbana, IL 61801, USA; [email protected]) Omslag met flap_MJOosterkamp.indd 1 25-11-2013 5:58:31 Physiology and biochemistry of aromatic hydrocarbon-degrading bacteria that use chlorate and/or nitrate as electron acceptor Margreet J. Oosterkamp Thesis-MJOosterkamp.indd 1 25-11-2013 6:42:09 Thesis committee Thesis supervisor Prof. dr. ir. A. J. M. Stams Personal Chair at the Laboratory of Microbiology Wageningen University Thesis co-supervisors Dr. C. M. Plugge Assistant Professor at the Laboratory of Microbiology Wageningen University Dr. P. J. Schaap Assistant Professor at the Laboratory of Systems and Synthetic Biology Wageningen University Other members Prof. dr. L. Dijkhuizen, University of Groningen Prof. dr. H. J. Laanbroek, University of Utrecht Prof. -

Investigation of a Sulfur-Utilizing Perchlorate-Reducing Bacterial Consortium" (2011)
University of Massachusetts Amherst ScholarWorks@UMass Amherst Open Access Dissertations 5-13-2011 Investigation of a Sulfur-Utilizing Perchlorate- Reducing Bacterial Consortium Teresa Anne Conneely University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/open_access_dissertations Part of the Bacteriology Commons Recommended Citation Conneely, Teresa Anne, "Investigation of a Sulfur-Utilizing Perchlorate-Reducing Bacterial Consortium" (2011). Open Access Dissertations. 372. https://scholarworks.umass.edu/open_access_dissertations/372 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. INVESTIGATION OF A SULFUR-UTILIZING PERCHLORATE-REDUCING BACTERIAL CONSORTIUM A Dissertation Presented by TERESA ANNE CONNEELY Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY May 2011 Department of Microbiology © Copyright by Teresa Anne Conneely 2011 All Rights Reserved INVESTIGATION OF A SULFUR-UTILIZING PERCHLORATE-REDUCING BACTERIAL CONSORTIUM A Dissertation Presented by TERESA ANNE CONNEELY Approved as to style and content by: ____________________________________ Klaus Nüsslein, Chair ____________________________________ Jeffery Blanchard, Member ____________________________________ James F. Holden, Member ____________________________________ Sarina Ergas, Member __________________________________________ John Lopes, Department Head Department of Microbiology DEDICATION Lé grá dó mo chlann ACKNOWLEDGMENTS I would like to thank my advisor Klaus Nüsslein for inviting me to join his lab, introducing me to microbial ecology and guiding me on my research path and goal of providing clean water through microbiology. Thank you to all my committee members throughout my Ph.D. -

Structure and Evolution of Chlorate Reduction Composite Transposons
RESEARCH ARTICLE Structure and Evolution of Chlorate Reduction Composite Transposons Iain C. Clark,a Ryan A. Melnyk,b Anna Engelbrektson,b John D. Coatesb Department of Civil and Environmental Engineeringa and Department of Plant and Microbial Biology,b University of California, Berkeley, California, USA ABSTRACT The genes for chlorate reduction in six bacterial strains were analyzed in order to gain insight into the metabolism. A newly isolated chlorate-reducing bacterium (Shewanella algae ACDC) and three previously isolated strains (Ideonella dechlora- tans, Pseudomonas sp. strain PK, and Dechloromarinus chlorophilus NSS) were genome sequenced and compared to published sequences (Alicycliphilus denitrificans BC plasmid pALIDE01 and Pseudomonas chloritidismutans AW-1). De novo assembly of genomes failed to join regions adjacent to genes involved in chlorate reduction, suggesting the presence of repeat regions. Using a bioinformatics approach and finishing PCRs to connect fragmented contigs, we discovered that chlorate reduction genes are flanked by insertion sequences, forming composite transposons in all four newly sequenced strains. These insertion sequences delineate regions with the potential to move horizontally and define a set of genes that may be important for chlorate reduction. In addition to core metabolic components, we have highlighted several such genes through comparative analysis and visualiza- tion. Phylogenetic analysis places chlorate reductase within a functionally diverse clade of type II dimethyl sulfoxide (DMSO) reductases, part of a larger family of enzymes with reactivity toward chlorate. Nucleotide-level forensics of regions surrounding chlorite dismutase (cld), as well as its phylogenetic clustering in a betaproteobacterial Cld clade, indicate that cld has been mobi- lized at least once from a perchlorate reducer to build chlorate respiration. -

Microbial Degradation of Organic Micropollutants in Hyporheic Zone Sediments
Microbial degradation of organic micropollutants in hyporheic zone sediments Dissertation To obtain the Academic Degree Doctor rerum naturalium (Dr. rer. nat.) Submitted to the Faculty of Biology, Chemistry, and Geosciences of the University of Bayreuth by Cyrus Rutere Bayreuth, May 2020 This doctoral thesis was prepared at the Department of Ecological Microbiology – University of Bayreuth and AG Horn – Institute of Microbiology, Leibniz University Hannover, from August 2015 until April 2020, and was supervised by Prof. Dr. Marcus. A. Horn. This is a full reprint of the dissertation submitted to obtain the academic degree of Doctor of Natural Sciences (Dr. rer. nat.) and approved by the Faculty of Biology, Chemistry, and Geosciences of the University of Bayreuth. Date of submission: 11. May 2020 Date of defense: 23. July 2020 Acting dean: Prof. Dr. Matthias Breuning Doctoral committee: Prof. Dr. Marcus. A. Horn (reviewer) Prof. Harold L. Drake, PhD (reviewer) Prof. Dr. Gerhard Rambold (chairman) Prof. Dr. Stefan Peiffer In the battle between the stream and the rock, the stream always wins, not through strength but by perseverance. Harriett Jackson Brown Jr. CONTENTS CONTENTS CONTENTS ............................................................................................................................ i FIGURES.............................................................................................................................. vi TABLES .............................................................................................................................. -
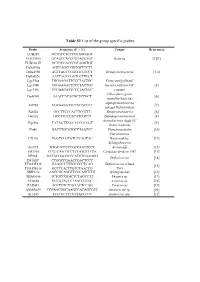
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

International Journal of Systematic and Evolutionary Microbiology
University of Plymouth PEARL https://pearl.plymouth.ac.uk 01 University of Plymouth Research Outputs University of Plymouth Research Outputs 2017-05-01 Reclassification of Thiobacillus aquaesulis (Wood & Kelly, 1995) as Annwoodia aquaesulis gen. nov., comb. nov., transfer of Thiobacillus (Beijerinck, 1904) from the Hydrogenophilales to the Nitrosomonadales, proposal of Hydrogenophilalia class. nov. within the 'Proteobacteria', and four new families within the orders Nitrosomonadales and Rhodocyclales Boden, R http://hdl.handle.net/10026.1/8740 10.1099/ijsem.0.001927 International Journal of Systematic and Evolutionary Microbiology All content in PEARL is protected by copyright law. Author manuscripts are made available in accordance with publisher policies. Please cite only the published version using the details provided on the item record or document. In the absence of an open licence (e.g. Creative Commons), permissions for further reuse of content should be sought from the publisher or author. International Journal of Systematic and Evolutionary Microbiology Reclassification of Thiobacillus aquaesulis (Wood & Kelly, 1995) as Annwoodia aquaesulis gen. nov., comb. nov. Transfer of Thiobacillus (Beijerinck, 1904) from the Hydrogenophilales to the Nitrosomonadales, proposal of Hydrogenophilalia class. nov. within the 'Proteobacteria', and 4 new families within the orders Nitrosomonadales and Rhodocyclales. --Manuscript Draft-- Manuscript Number: IJSEM-D-16-00980R2 Full Title: Reclassification of Thiobacillus aquaesulis (Wood & Kelly, -

Isolation and Characterization of ISA Degrading Alkaliphilic Bacteria
Isolation and Characterization of ISA Degrading Alkaliphilic Bacteria Zohier Salah (Researcher) A thesis submitted to the University of Huddersfield in the partial fulfilment of the requirements for the degree of Doctor of Philosophy School of Applied Science December 2017 Acknowledgment Praise be to Allah through whose mercy (and favors) all good things are accomplished Firstly, I would like to express my sincere gratitude to my main supervisor Professor Paul N. Humphreys who gave me the opportunity to work with him and for the continuous support of my PhD study and related research, for his patience, motivation, and immense knowledge. His guidance helped me in all the time of research and writing of this thesis. Also, I wish to express my appreciation to my second supervisor, Professor Andy Laws for all the assistance he offered. Without they precious support it would not be possible to conduct this research. Special thanks go to the Government of Libya for providing me with the financial support for this study. I would also like to thank all the laboratory support staff in the School of Applied Sciences, University of Huddersfield, for all the support. Sincerely appreciations also go to my family especially, my parents and my virtuous wife Zainab, my son Mohamed and the extended my family, especially my brother Mr. Ahmed Salah. Finally, but by no means least, thanks to all my colleagues in the research group who helped with experience, ideas and discussions during my studies, Dr Simon P Rout, Dr Isaac A Kyeremeh, Dr Christopher J Charles and to all who contributed in diverse ways to make my research at the University of Huddersfield a success. -

Species-Level Microbiome Composition of Activated Sludge - Introducing the Midas 3 2 Ecosystem-Specific Reference Database and Taxonomy
bioRxiv preprint doi: https://doi.org/10.1101/842393; this version posted November 14, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Species-level microbiome composition of activated sludge - introducing the MiDAS 3 2 ecosystem-specific reference database and taxonomy 3 Marta Nierychlo, Kasper Skytte Andersen, Yijuan Xu, Nick Green, Mads Albertsen, Morten S. 4 Dueholm, Per Halkjær Nielsen. 5 Center for Microbial Communities, Department of Chemistry and Bioscience, Aalborg University, 6 Aalborg, Denmark. 7 *Correspondence to: Per Halkjær Nielsen, Center for Microbial Communities, Department of 8 Chemistry and Bioscience, Aalborg University, Fredrik Bajers Vej 7H, 9220 Aalborg, Denmark; 9 Phone: +45 9940 8503; Fax: Not available; E-mail: [email protected] 10 Abstract 11 The function of microbial communities in wastewater treatment systems and anaerobic digesters is 12 dictated by the physiological activity of its members and complex interactions between them. Since 13 functional traits are often conserved at low taxonomic ranks (genus, species, strain), the development 14 of high taxonomic resolution and reliable classification is the first crucial step towards understanding 15 the role of microbes in any ecosystem. Here we present MiDAS 3, a comprehensive 16S rRNA gene 16 reference database based on high-quality full-length sequences derived from activated sludge and 17 anaerobic digester systems. The MiDAS 3 taxonomy proposes unique provisional names for all 18 microorganisms down to species level.