Breeding Strategies of Open-Cup-Nesting Birds in Sub-Antarctic Forests of Navarino Island, Chile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
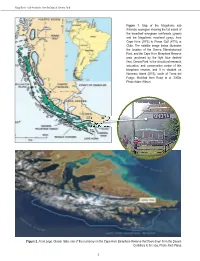
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

House Sparrow Eradication Attempt on Robinson Crusoe Island, Juan Fernández Archipelago, Chile
E. Hagen, J. Bonham and K. Campbell Hagen, E.; J. Bonham and K. Campbell. House sparrow eradication attempt on Robinson Crusoe Island, Juan Fernández Archipelago, Chile House sparrow eradication attempt on Robinson Crusoe Island, Juan Fernández Archipelago, Chile E. Hagen1, J. Bonham1 and K. Campbell1,2 1Island Conservation, Las Urbinas 53 Santiago, Chile. <[email protected]>.2School of Geography, Planning & Environmental Management, The University of Queensland, St Lucia 4072, Australia. Abstract House sparrows (Passer domesticus) compete with native bird species, consume crops, and are vectors for diseases in areas where they have been introduced. Sparrow eradication attempts aimed at eliminating these negative eff ects highlight the importance of deploying multiple alternative methods to remove individuals while maintaining the remaining population naïve to techniques. House sparrow eradication was attempted from Robinson Crusoe Island, Chile, in the austral winter of 2012 using an experimental approach sequencing passive multi-catch traps, passive single- catch traps, and then active multi-catch methods, and fi nally active single-catch methods. In parallel, multiple detection methods were employed and local stakeholders were engaged. The majority of removals were via passive trapping, and individuals were successfully targeted with active methods (mist nets and shooting). Automated acoustic recording, point counts and camera traps declined in power to detect individual sparrows as the population size decreased; however, we continued to detect sparrows at all population densities using visual observations, underscoring the importance of local residents’ participation in monitoring. Four surviving sparrows were known to persist at the conclusion of eff orts in 2012. Given the lack of formal biosecurity measures within the Juan Fernández archipelago, reinvasion is possible. -

Argentine and Chilean Claims to British Antarctica. - Bases Established in the South Shetlands
Keesing's Record of World Events (formerly Keesing's Contemporary Archives), Volume VI-VII, February, 1948 Argentine, Chilean, British, Page 9133 © 1931-2006 Keesing's Worldwide, LLC - All Rights Reserved. Argentine and Chilean Claims to British Antarctica. - Bases established in the South Shetlands. - Chilean President inaugurates Chilean Army Bases on Greenwich Island. - Argentine Naval Demonstration in British Antarctic Waters. - H.M.S. "Nigeria" despatched to Falklands. - British Government Statements. - Argentine-Chilean Agreement on Joint Defence of "Antarctic Rights." - The Byrd and Ronne Antarctic Expeditions. - Australian Antarctic Expedition occupies Heard Islands. The Foreign-Office in London, in statements on Feb. 7 and Feb. 13, announced that Argentina and Chile had rejected British protests, earlier presented in Buenos Aires and Santiago, against the action of those countries in establishing bases in British Antarctic territories. The announcement of Feb. 7 stated that on Dec. 7, 1947, the British Ambassador in Buenos Aires, Sir Reginald Leeper, had presented a Note expressing British "anxiety" at the activities in the Antarctic of an Argentine naval expedition which had visited part of the Falkland Islands Dependencies, including Graham Land, the South Shetlands, and the South Orkneys, and had landed at various points in British territory; that a request had been made for Argentine nationals to evacuate bases established on Deception Island and Gamma Island, in the South Shetlands; that H.M. Government had proposed that the Argentine should submit her claim to Antarctic sovereignty to the International Court of Justice for adjudication; and that on Dec. 23, 1947, a second British Note had been presented expressing surprise at continued violations of British territory and territorial waters by Argentine vessels in the Antarctic. -

Composition and Structure of Bird Flocks in a Temperate Forest of Central Chile
Revista Chilena de Ornitología 26(1): 33-36 Unión de Ornitólogos de Chile 2020 COMUNICACIÓN BREVE 33 COMPOSITION AND STRUCTURE OF BIRD FLOCKS IN A TEMPERATE FOREST OF CENTRAL CHILE Composición y estructura de bandadas en el bosque templado de Chile central FERNANDO MEDRANO1,2, MARÍA A. VUKASOVIC3,4, ROMINA CHIAPPE3,4 & CRISTIÁN F. ESTADES3,4 1Departamento de Biología Evolutiva, Ecologia i Ciències Ambientals, Institut de Recerca de la Biodiversitat (IRBio), Facultat de Biología, Universitat de Barcelona, Barcelona 08028, España. 2Red de Observadores de Aves y Vida Silvestre de Chile. Santiago, Chile. 3Unión de Ornitólogos de Chile (AvesChile). Santiago, Chile. 4Laboratorio de Ecología de Vida Silvestre. Facultad de Ciencias Forestales y Conservación de la Naturaleza. Santa Rosa #11315. La Pintana, Santiago, Chile. Correspondencia: F. Medrano, [email protected] RESUMEN.- Formar bandadas es una adaptación de las aves para aumentar la eficiencia de forrajeo y disminuir la depredación. Pese a que en bosques tropicales existen numerosos estudios sobre la formación de bandadas, en bosques templados de Sudamérica esta conducta ha sido escasamente documentada. En este estudio describi- mos la composición de 77 bandadas encontradas en un paisaje forestal de la región del Maule entre 1999-2002, y analizamos si existen relaciones entre las abundancias dentro de las bandadas entre especies. Usando simu- laciones de Monte Carlo analizamos la prevalencia de diferentes especies a formar bandadas y relacionamos la abundancia de las especies entre sí dentro de las bandadas. Cada bandada tuvo en promedio 10,5 individuos y la especie más frecuente fue Aphrastura spinicauda. Pese a que se ha señalado a A. -

Invaders Without Frontiers: Cross-Border Invasions of Exotic Mammals
Biological Invasions 4: 157–173, 2002. © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Review Invaders without frontiers: cross-border invasions of exotic mammals Fabian M. Jaksic1,∗, J. Agust´ın Iriarte2, Jaime E. Jimenez´ 3 & David R. Mart´ınez4 1Center for Advanced Studies in Ecology & Biodiversity, Pontificia Universidad Catolica´ de Chile, Casilla 114-D, Santiago, Chile; 2Servicio Agr´ıcola y Ganadero, Av. Bulnes 140, Santiago, Chile; 3Laboratorio de Ecolog´ıa, Universidad de Los Lagos, Casilla 933, Osorno, Chile; 4Centro de Estudios Forestales y Ambientales, Universidad de Los Lagos, Casilla 933, Osorno, Chile; ∗Author for correspondence (e-mail: [email protected]; fax: +56-2-6862615) Received 31 August 2001; accepted in revised form 25 March 2002 Key words: American beaver, American mink, Argentina, Chile, European hare, European rabbit, exotic mammals, grey fox, muskrat, Patagonia, red deer, South America, wild boar Abstract We address cross-border mammal invasions between Chilean and Argentine Patagonia, providing a detailed history of the introductions, subsequent spread (and spread rate when documented), and current limits of mammal invasions. The eight species involved are the following: European hare (Lepus europaeus), European rabbit (Oryctolagus cuniculus), wild boar (Sus scrofa), and red deer (Cervus elaphus) were all introduced from Europe (Austria, France, Germany, and Spain) to either or both Chilean and Argentine Patagonia. American beaver (Castor canadensis) and muskrat (Ondatra zibethicus) were introduced from Canada to Argentine Tierra del Fuego Island (shared with Chile). The American mink (Mustela vison) apparently was brought from the United States of America to both Chilean and Argentine Patagonia, independently. The native grey fox (Pseudalopex griseus) was introduced from Chilean to Argentine Tierra del Fuego. -

MORPHOLOGICAL and ECOLOGICAL EVOLUTION in OLD and NEW WORLD FLYCATCHERS a Dissertation Presented to the Faculty of the College O
MORPHOLOGICAL AND ECOLOGICAL EVOLUTION IN OLD AND NEW WORLD FLYCATCHERS A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Clay E. Corbin August 2002 This dissertation entitled MORPHOLOGICAL AND ECOLOGICAL EVOLUTION IN OLD AND NEW WORLD FLYCATCHERS BY CLAY E. CORBIN has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Donald B. Miles Associate Professor, Department of Biological Sciences Leslie A. Flemming Dean, College of Arts and Sciences CORBIN, C. E. Ph.D. August 2002. Biological Sciences. Morphological and Ecological Evolution in Old and New World Flycatchers (215pp.) Director of Dissertation: Donald B. Miles In both the Old and New Worlds, independent clades of sit-and-wait insectivorous birds have evolved. These independent radiations provide an excellent opportunity to test for convergent relationships between morphology and ecology at different ecological and phylogenetic levels. First, I test whether there is a significant adaptive relationship between ecology and morphology in North American and Southern African flycatcher communities. Second, using morphological traits and observations on foraging behavior, I test whether ecomorphological relationships are dependent upon locality. Third, using multivariate discrimination and cluster analysis on a morphological data set of five flycatcher clades, I address whether there is broad scale ecomorphological convergence among flycatcher clades and if morphology predicts a course measure of habitat preference. Finally, I test whether there is a common morphological axis of diversification and whether relative age of origin corresponds to the morphological variation exhibited by elaenia and tody-tyrant lineages. -

First Evidence of Insect Attraction by a Southern Hemisphere Splachnaceae: the Case of Tayloria Dubyi Broth
Nova Hedwigia Vol. 92 issue 3–4, 317–326 Article Stuttgart, May 2011 First evidence of insect attraction by a Southern Hemisphere Splachnaceae: The case of Tayloria dubyi Broth. in the Reserve Biosphere Cape Horn, Chile. Jocelyn Jofre1*, Bernard Goffinet3, Paul Marino4, Robert A. Raguso5, Silvio Shigueo Nihei6, Francisca Massardo1,2 and Ricardo Rozzi1,2,7 1 Instituto de Ecología y Biodiversidad, Facultad de Ciencias, Universidad de Chile, Casilla 653, Santiago, Chile. [email protected] 2 Programa de Magíster en Ciencias, Facultad de Ciencias, Universidad de Magallanes, Casilla 113-D, Avenida Bulnes 01855, Punta Arenas, Chile 3 Department of Ecology and Evolutionary Biology, 75 N. Eagleville Road, University of Connecticut, Storrs, CT 06269-3043, USA 4 Department of Biology, Memorial University, St. John´s, NL A1B 3X9, Canada 5 Department of Neurobiology and Behavior, Seeley G. Mudd Hall, Cornell University, Ithaca, NY 14853-2702, USA 6 Departamento de Zoologia, Instituto de Biociências – Universidade de São Paulo, Rua do Matão, Trav. 14, n. 101, 05508-900, São Paulo/SP, Brasil. 7 Department of Philosophy, University of North Texas, Denton, TX 76201 With 3 figures and 1 table Jofre, J., B. Goffinet, P. Marino, R.A. Raguso, S.S. Nihei, F. Massardo & R. Rozzi (2011): First evidence of insect attraction by a Southern Hemisphere Splachnaceae: The case of Tayloria dubyi Broth. in the Reserve Biosphere Cape Horn, Chile. – Nova Hedwigia 92: 317–326. Abstract: The moss Tayloria dubyi (Splachnaceae) is endemic to the subantarctic Magallanes ecoregion where it grows exclusively on bird dung and perhaps only on feces of the goose Chloephaga picta, a unique habitat among Splachnaceae. -

With National Geographic
Around Cape Horn & the Chilean Fjords - with National Geographic From 02/03/2021 From Ushuaia Ship: LE BOREAL to 14/03/2021 to Talcahuano In partnership with National Geographic Expeditions. Discover Patagonia during a 13-day expedition cruise. PONANT is offering you the opportunity to explore the most beautiful scenery Argentina and Chile have to offer, including glaciers, fjords, and winding channels. You will embark in Ushuaia, the world’s southernmost city, and round the mythical Cape Horn. Puerto Williams, considerated as the southernmost village in the world, will be your first port of call. Next enter the magic of the Chilean fjords and channels for exceptional moments spent sailing amidst landscapes of unrivalled beauty. The narrow passages, channels and fjords will lead you right up close to stunning glaciers, including Garibaldi, Agostini, El Brujo, Overnight in Buenos Aires + flight Buenos Pie XI. Aires/Ushuaia + visit + flight Concepcion/Santiago Your ship will reach Tortel and its charming stilt houses interconnected by a labyrinth of wooden footbridges. During the last part of your cruise in South America, you will call at Quemchi, after a sailing during which it is not unusual to encounter sea lions and porpoises. Quemchi is an authentic village located on the lush island of Chiloé. Subject to ice and weather conditions. The expedition highlights and itineraries described above illustrate possible experiences only and cannot be guaranteed. The information in this document is valid as of 11/08/2020 Around Cape Horn & the Chilean Fjords - with National Geographic YOUR STOPOVERS : USHUAIA Embarkation 02/03/2021 from 16h00 to 17h00 Departure 02/03/2021 at 18h00 Capital of Argentina's Tierra del Fuego province, Ushuaia is considered the gateway to the White Continent and the South Pole. -

Dientes De Navarino Circuit TREK 7 Days Wilderness Trekking on the Isla Navarino - Tierra Del Fuego
Dientes de Navarino Circuit TREK 7 days Wilderness Trekking on the Isla Navarino - Tierra del Fuego The “Dientes de Navarino Circuit” is a trekking experience at the edge of the world! This southernmost trekking route is a once-in-a-lifetime experience that will fascinate curious and experienced hikers. On this pioneer-adventure in the mountains of Dientes de Navarino, you will land right in the middle of Tierra del Fuego’s mystic beauty. Hike through wet swampland and quaint forests of beech trees before you finally reach the sharp teeth, Los Dientes, of Navarino Island. These natural jewels are embedded in small blue-lustrous lagoons. Also hike through the southern end of the world to watch beavers building their dams, while resisting the extremely strong gusts of wind that indicate the closeness of Cape Horn. Trip Highlights: • Hike the southernmost trek at the edge of the world, the Dientes de Navarino Circuit on Isla Navarino • Set up camp between rock pinnacles, alpine lakes & beaver dams • Visit the charming fishing village Puerto Williams, the most southerly village in the world • Fly over the legendary Strait of Magellan, Darwin mountain range and Beagle-Channel • Enjoy a freshly caught king crab prepared by a local family Insider Tip: • Take a trip from Ushuaia to Martillo Island and learn more about see lions, dolphins and Magellan penguins Trip Info: Trip length: 7 days Start/End of the trip: from / to Punta Arenas or Ushuaia Group Size: min. 3 / max. 12 people Departures: see set departures on our website or individual on -

Bird Conservation International Molecular Characterisation of Haemoparasites in Forest Birds from Robinson Crusoe Island: Is
Bird Conservation International http://journals.cambridge.org/BCI Additional services for Bird Conservation International: Email alerts: Click here Subscriptions: Click here Commercial reprints: Click here Terms of use : Click here Molecular characterisation of haemoparasites in forest birds from Robinson Crusoe Island: Is the Austral Thrush a potential threat to endemic birds? JAVIER MARTÍNEZ, RODRIGO A. VÁSQUEZ, CRISTOBAL VENEGAS and SANTIAGO MERINO Bird Conservation International / Volume 25 / Issue 02 / June 2015, pp 139 - 152 DOI: 10.1017/S0959270914000227, Published online: 19 August 2014 Link to this article: http://journals.cambridge.org/abstract_S0959270914000227 How to cite this article: JAVIER MARTÍNEZ, RODRIGO A. VÁSQUEZ, CRISTOBAL VENEGAS and SANTIAGO MERINO (2015). Molecular characterisation of haemoparasites in forest birds from Robinson Crusoe Island: Is the Austral Thrush a potential threat to endemic birds?. Bird Conservation International, 25, pp 139-152 doi:10.1017/S0959270914000227 Request Permissions : Click here Downloaded from http://journals.cambridge.org/BCI, IP address: 212.128.9.27 on 22 May 2015 Bird Conservation International (2015) 25 :139 –152 . © BirdLife International, 2014 doi:10.1017/S0959270914000227 Molecular characterisation of haemoparasites in forest birds from Robinson Crusoe Island: Is the Austral Thrush a potential threat to endemic birds? JAVIER MARTÍNEZ , RODRIGO A. VÁSQUEZ , CRISTOBAL VENEGAS and SANTIAGO MERINO Summary The Juan Fernández Firecrown Sephanoides fernandensis and Juan Fernández Tit-Tyrant Anairetes fernandezianus are two endemic forest birds inhabiting Robinson Crusoe Island and are classified as ‘Critically Endangered’ and ‘Near Threatened’ respectively by IUCN. Previous research concluded that the two main factors involved in the decline of these birds were habitat degradation and the introduction of predator / competitor species. -

Curriculum Vitae Abreviado
ABRIDGED CURRICULUM VITAE - March 2016 1. PERSONAL DATA Name, Christian names: RABASSA, Jorge Oscar Born in: La Plata, Argentina, on December 2nd. 1948 Argentine Passport N°: 05.400.236 M Home Street: Buenos Aires 2869, Barrio Los Alakalufes 2. City: V9410BFD. USHUAIA Province: Tierra del Fuego, Argentina Mobile telephone: +54-9-2901-6-19503 Office Phone + Fax: +54-2901-430644/422310/422314 2. EDUCATION Licenciado en Geología, 20/08/71. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Argentina. Doctor en Ciencias Naturales (Geology), 23/12/74. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata. 3. POSITIONS HELD IN EDUCATION AND RESEARCH Research and Science Management Director of CADIC, CONICET, from October 27th., 2011, onwards. Investigador Superior, CADIC, CONICET, from 1-10-10 up to date. President, Argentine Association of Geomorphology and Quaternary Studies (2015- 2018; affiliated to INQUA and IAG) Investigador Principal, CADIC, CONICET, 1-1-95 al 1-10-10. Minister of Education, Culture, Science and Technology, province of Tierra del Fuego, December 2007 - june 2008. Member of the Earth Sciences Committee, CONICET, april 2006 - november 2007, and 2004. Rector, Universidad Nacional del Comahue. 1998-2002 Investigador Independiente, CADIC, CONICET, 1988-1994. Director, CADIC, Centro Austral de Investigaciones Científicas, CONICET, Ushuaia, 1986-1990. Investigador Independiente, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, 1985-1988. Investigador Principal, Instituto Antártico Argentino, Programa HIELOANTAR, Proyecto GEOGLA-Ross, Summer seasons 1980-1981-1982. Investigador Adjunto, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, 1979-1985. Fulbright Post-doctoral Fellow and Senior Research Associate, Department of Geological Sciences, State University of New York at Binghamton, Binghamton, New York, U.S.A., 1975-1976. -

Yaghan's, Explorers and Settlers
YAGHAN’S, EXPLORERS AND SETTLERS: 10,000 years of Southern Tierra del Fuego Archipelago History The Museum Permanent Exhibit Script Martin Gusinde Anthropological Museum · Puerto Williams - Chile Participants in Initiation Rite in the year 1922. (Martin Gusinde, last row, fourth position from left to right). Anthropos Institut. Sankt Augustin, Germany - Authorized Digital Copying Martin Gusinde Anthropological Museum Introduction INTROUDUCTION The creation of a museum on Navarino Island was an ambitious project that grew out of a deep interest in and concern for the island’s natural and cultural heritage. The initiative was originally proposed by the Chilean Navy, the same institution that built the Martín Gusinde Anthropological Museum (MAMG), which opened its doors in Puerto Williams in 1975. In the 1960s, before the museum was founded, a collection was begun of archeological material from the island’s coastal areas, along with some objects of historical interest from the first occupation by pioneers. This collection was exhibited in the now defunct Mixed School Nº in Puerto Williams. The collection was moved when the Martin Gusinde Museum was established, becoming part of that institution permanent collection. The Museum was named after the Austrian anthropologist and priest Martin Gusinde S.V.D. (1886-1969), who worked among the Yaghan and Selk’nam people from 1918 to 1924. His body of work offers the greatest collection of ethnographic studies about a world that was already on the verge of disappearing. His seminal work “The Indians of Tierra del Fuego” was published between 190 and 1974, and will forever remain the principal source of information we possess about the native people of Tierra del Fuego.