Chironomidae from Ethiopia. Part 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chironomus Frontpage No 28
CHIRONOMUS Journal of Chironomidae Research No. 30 ISSN 2387-5372 December 2017 CONTENTS Editorial Anderson, A.M. Keep the fuel burning 2 Current Research Epler, J. An annotated preliminary list of the Chironomidae of Zurqui 4 Martin, J. Chironomus strenzkei is a junior synonym of C. stratipennis 19 Andersen, T. et al. Two new Neo- tropical Chironominae genera 26 Kuper, J. Life cycle of natural populations of Metriocnemus (Inermipupa) carmencitabertarum in The Netherlands: indications for a southern origin 55 Lin, X., Wang, X. A redescription of Zavrelia bragremia 67 Short Communications Baranov, V., Nekhaev, I. Impact of the bird-manure caused eutrophication on the abundance and diversity of chironomid larvae in lakes of the Bolshoy Aynov Island 72 Namayandeh, A., Beresford, D.V. New range extensions for the Canadian Chironomidae fauna from two urban streams 76 News Liu, W. et al. The 2nd Chinese Symposium on Chironomidae 81 In memoriam Michailova, P., et al. Prof. Dr. Wolfgang Friedrich Wülker 82 Unidentified male, perhaps of the Chironomus decorus group? Photo taken in the Madrona Marsh Preserve, California, USA. Photo: Emile Fiesler. CHIRONOMUS Journal of Chironomidae Research Editors Alyssa M. ANDERSON, Department of Biology, Chemistry, Physics, and Mathematics, Northern State University, Aberdeen, South Dakota, USA. Torbjørn EKREM, NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway. Peter H. LANGTON, 16, Irish Society Court, Coleraine, Co. Londonderry, Northern Ireland BT52 1GX. The CHIRONOMUS Journal of Chironomidae Research is devoted to all aspects of chironomid research and serves as an up-to-date research journal and news bulletin for the Chironomidae research community. -

Is Ellipura Monophyletic? a Combined Analysis of Basal Hexapod
ARTICLE IN PRESS Organisms, Diversity & Evolution 4 (2004) 319–340 www.elsevier.de/ode Is Ellipura monophyletic? A combined analysis of basal hexapod relationships with emphasis on the origin of insects Gonzalo Giribeta,Ã, Gregory D.Edgecombe b, James M.Carpenter c, Cyrille A.D’Haese d, Ward C.Wheeler c aDepartment of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, 16 Divinity Avenue, Cambridge, MA 02138, USA bAustralian Museum, 6 College Street, Sydney, New South Wales 2010, Australia cDivision of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, USA dFRE 2695 CNRS, De´partement Syste´matique et Evolution, Muse´um National d’Histoire Naturelle, 45 rue Buffon, F-75005 Paris, France Received 27 February 2004; accepted 18 May 2004 Abstract Hexapoda includes 33 commonly recognized orders, most of them insects.Ongoing controversy concerns the grouping of Protura and Collembola as a taxon Ellipura, the monophyly of Diplura, a single or multiple origins of entognathy, and the monophyly or paraphyly of the silverfish (Lepidotrichidae and Zygentoma s.s.) with respect to other dicondylous insects.Here we analyze relationships among basal hexapod orders via a cladistic analysis of sequence data for five molecular markers and 189 morphological characters in a simultaneous analysis framework using myriapod and crustacean outgroups.Using a sensitivity analysis approach and testing for stability, the most congruent parameters resolve Tricholepidion as sister group to the remaining Dicondylia, whereas most suboptimal parameter sets group Tricholepidion with Zygentoma.Stable hypotheses include the monophyly of Diplura, and a sister group relationship between Diplura and Protura, contradicting the Ellipura hypothesis.Hexapod monophyly is contradicted by an alliance between Collembola, Crustacea and Ectognatha (i.e., exclusive of Diplura and Protura) in molecular and combined analyses. -

Ohio EPA Macroinvertebrate Taxonomic Level December 2019 1 Table 1. Current Taxonomic Keys and the Level of Taxonomy Routinely U
Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Table 1. Current taxonomic keys and the level of taxonomy routinely used by the Ohio EPA in streams and rivers for various macroinvertebrate taxonomic classifications. Genera that are reasonably considered to be monotypic in Ohio are also listed. Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Species Pennak 1989, Thorp & Rogers 2016 Porifera If no gemmules are present identify to family (Spongillidae). Genus Thorp & Rogers 2016 Cnidaria monotypic genera: Cordylophora caspia and Craspedacusta sowerbii Platyhelminthes Class (Turbellaria) Thorp & Rogers 2016 Nemertea Phylum (Nemertea) Thorp & Rogers 2016 Phylum (Nematomorpha) Thorp & Rogers 2016 Nematomorpha Paragordius varius monotypic genus Thorp & Rogers 2016 Genus Thorp & Rogers 2016 Ectoprocta monotypic genera: Cristatella mucedo, Hyalinella punctata, Lophopodella carteri, Paludicella articulata, Pectinatella magnifica, Pottsiella erecta Entoprocta Urnatella gracilis monotypic genus Thorp & Rogers 2016 Polychaeta Class (Polychaeta) Thorp & Rogers 2016 Annelida Oligochaeta Subclass (Oligochaeta) Thorp & Rogers 2016 Hirudinida Species Klemm 1982, Klemm et al. 2015 Anostraca Species Thorp & Rogers 2016 Species (Lynceus Laevicaudata Thorp & Rogers 2016 brachyurus) Spinicaudata Genus Thorp & Rogers 2016 Williams 1972, Thorp & Rogers Isopoda Genus 2016 Holsinger 1972, Thorp & Rogers Amphipoda Genus 2016 Gammaridae: Gammarus Species Holsinger 1972 Crustacea monotypic genera: Apocorophium lacustre, Echinogammarus ischnus, Synurella dentata Species (Taphromysis Mysida Thorp & Rogers 2016 louisianae) Crocker & Barr 1968; Jezerinac 1993, 1995; Jezerinac & Thoma 1984; Taylor 2000; Thoma et al. Cambaridae Species 2005; Thoma & Stocker 2009; Crandall & De Grave 2017; Glon et al. 2018 Species (Palaemon Pennak 1989, Palaemonidae kadiakensis) Thorp & Rogers 2016 1 Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Informal grouping of the Arachnida Hydrachnidia Smith 2001 water mites Genus Morse et al. -

Checklist of the Family Chironomidae (Diptera) of Finland
A peer-reviewed open-access journal ZooKeys 441: 63–90 (2014)Checklist of the family Chironomidae (Diptera) of Finland 63 doi: 10.3897/zookeys.441.7461 CHECKLIST www.zookeys.org Launched to accelerate biodiversity research Checklist of the family Chironomidae (Diptera) of Finland Lauri Paasivirta1 1 Ruuhikoskenkatu 17 B 5, FI-24240 Salo, Finland Corresponding author: Lauri Paasivirta ([email protected]) Academic editor: J. Kahanpää | Received 10 March 2014 | Accepted 26 August 2014 | Published 19 September 2014 http://zoobank.org/F3343ED1-AE2C-43B4-9BA1-029B5EC32763 Citation: Paasivirta L (2014) Checklist of the family Chironomidae (Diptera) of Finland. In: Kahanpää J, Salmela J (Eds) Checklist of the Diptera of Finland. ZooKeys 441: 63–90. doi: 10.3897/zookeys.441.7461 Abstract A checklist of the family Chironomidae (Diptera) recorded from Finland is presented. Keywords Finland, Chironomidae, species list, biodiversity, faunistics Introduction There are supposedly at least 15 000 species of chironomid midges in the world (Armitage et al. 1995, but see Pape et al. 2011) making it the largest family among the aquatic insects. The European chironomid fauna consists of 1262 species (Sæther and Spies 2013). In Finland, 780 species can be found, of which 37 are still undescribed (Paasivirta 2012). The species checklist written by B. Lindeberg on 23.10.1979 (Hackman 1980) included 409 chironomid species. Twenty of those species have been removed from the checklist due to various reasons. The total number of species increased in the 1980s to 570, mainly due to the identification work by me and J. Tuiskunen (Bergman and Jansson 1983, Tuiskunen and Lindeberg 1986). -

Table of Contents 2
Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States including Standard Taxonomic Effort Levels 1 March 2011 Austin Brady Richards and D. Christopher Rogers Table of Contents 2 1.0 Introduction 4 1.1 Acknowledgments 5 2.0 Standard Taxonomic Effort 5 2.1 Rules for Developing a Standard Taxonomic Effort Document 5 2.2 Changes from the Previous Version 6 2.3 The SAFIT Standard Taxonomic List 6 3.0 Methods and Materials 7 3.1 Habitat information 7 3.2 Geographic Scope 7 3.3 Abbreviations used in the STE List 8 3.4 Life Stage Terminology 8 4.0 Rare, Threatened and Endangered Species 8 5.0 Literature Cited 9 Appendix I. The SAFIT Standard Taxonomic Effort List 10 Phylum Silicea 11 Phylum Cnidaria 12 Phylum Platyhelminthes 14 Phylum Nemertea 15 Phylum Nemata 16 Phylum Nematomorpha 17 Phylum Entoprocta 18 Phylum Ectoprocta 19 Phylum Mollusca 20 Phylum Annelida 32 Class Hirudinea Class Branchiobdella Class Polychaeta Class Oligochaeta Phylum Arthropoda Subphylum Chelicerata, Subclass Acari 35 Subphylum Crustacea 47 Subphylum Hexapoda Class Collembola 69 Class Insecta Order Ephemeroptera 71 Order Odonata 95 Order Plecoptera 112 Order Hemiptera 126 Order Megaloptera 139 Order Neuroptera 141 Order Trichoptera 143 Order Lepidoptera 165 2 Order Coleoptera 167 Order Diptera 219 3 1.0 Introduction The Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) is charged through its charter to develop standardized levels for the taxonomic identification of aquatic macroinvertebrates in support of bioassessment. This document defines the standard levels of taxonomic effort (STE) for bioassessment data compatible with the Surface Water Ambient Monitoring Program (SWAMP) bioassessment protocols (Ode, 2007) or similar procedures. -

Gastropoda: Opisthobranchia)
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Fall 1977 A MONOGRAPHIC STUDY OF THE NEW ENGLAND CORYPHELLIDAE (GASTROPODA: OPISTHOBRANCHIA) ALAN MITCHELL KUZIRIAN Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation KUZIRIAN, ALAN MITCHELL, "A MONOGRAPHIC STUDY OF THE NEW ENGLAND CORYPHELLIDAE (GASTROPODA: OPISTHOBRANCHIA)" (1977). Doctoral Dissertations. 1169. https://scholars.unh.edu/dissertation/1169 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Introducing the First Karyotype from the Genus Paramerina of the Subfamily Tanypodinae (Diptera: Chironomidae)
Journal of Entomology and Zoology Studies 2017; 5(6): 861-866 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Introducing the first karyotype from the genus JEZS 2017; 5(6): 861-866 © 2017 JEZS Paramerina of the subfamily Tanypodinae Received: 28-09-2017 Accepted: 29-10-2017 (Diptera: Chironomidae) A Maddi Reddy Department of Zoology, Bangalore University, A Maddi Reddy and S Ramakrishna Bengaluru, Karnataka, India Abstract S Ramakrishna The first karyotype of an unspecified species (2n=12) from the genus Paramerina from the subfamily Department of Zoology, Bangalore University, Tanypodinae collected from the south Indian geographical localities is presented. A tentative polytene Bengaluru, Karnataka, India chromosomal map was prepared and also the karyotype was prepared from somatic metaphase complement. The basic chromosomal number has been deduced from the karyotypes. Chromosomal descriptions also involve the patterns of C- bands and NOR localization. Head capsule morphology was used to arrive at the genus level identifications. Morphology of the shortest pompon-like chromosome has been discussed. Keywords: Tanypodinae, karyotype, Polytene chromosomes, differential replication, pompon Introduction The subfamily Tanypodinae under the family Chironomidae includes about 673 species of 58 genera worldwide. The genus Paramerina is distributed worldwide comprising about Twenty- eight species [29, 4, 25, 1, 31, 36, 30, 10, 5, 24, 26, 11] four species are known to be described in India [4, 11]. The polytene chromosomes of the subfamily Tanypodinae range from 2n=6 to 2n=16 in number [27]. The salivary glands possess cells of different sizes that contain thin long globular polytene chromosomes that have low degree of polyteny, appearing as loose amorphous structures hence stain poorly, which makes their banding pattern indistinct and difficult to analyze [43]. -

Bibliograp Bibliography
BIBLIOGRAPHY 9.1 BIBLIOGRAPHY 9 Adam, J.I & O.A. Sæther. 1999. Revision of the records for the southern United States genus Nilothauma Kieffer, 1921 (Diptera: (Diptera: Chironomidae). J. Ga. Ent. Soc. Chironomidae). Ent. scand. Suppl. 56: 1- 15:69-73. 107. Beck, W.M., Jr. & E.C. Beck. 1966. Chironomidae Ali, A. 1991. Perspectives on management of pest- (Diptera) of Florida - I. Pentaneurini (Tany- iferous Chironomidae (Diptera), an emerg- podinae). Bull. Fla. St. Mus. Biol. Sci. ing global problem. J. Am. Mosq. Control 10:305-379. Assoc. 7: 260-281. Beck, W.M., Jr., & E.C. Beck. 1970. The immature Armitage, P., P.S. Cranston & L.C.V. Pinder (eds). stages of some Chironomini (Chiro- 1995. The Chironomidae. Biology and nomidae). Q.J. Fla. Acad. Sci. 33:29-42. ecology of non-biting midges. Chapman & Bilyj, B. 1984. Descriptions of two new species of Hall, London. 572 pp. Tanypodinae (Diptera: Chironomidae) from Ashe, P. 1983. A catalogue of chironomid genera Southern Indian Lake, Canada. Can. J. Fish. and subgenera of the world including syn- Aquat. Sci. 41: 659-671. onyms (Diptera: Chironomidae). Ent. Bilyj, B. 1985. New placement of Tanypus pallens scand. Suppl. 17: 1-68. Coquillett, 1902 nec Larsia pallens (Coq.) Barton, D.R., D.R. Oliver & M.E. Dillon. 1993. sensu Roback 1971 (Diptera: Chironomi- The first Nearctic record of Stackelbergina dae) and redescription of the holotype. Can. Shilova and Zelentsov (Diptera: Chironomi- Ent. 117: 39-42. dae): Taxonomic and ecological observations. Bilyj, B. 1988. A taxonomic review of Guttipelopia Aquatic Insects 15: 57-63. (Diptera: Chironomidae). -
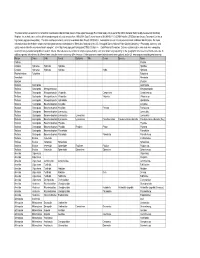
This Table Contains a Taxonomic List of Benthic Invertebrates Collected from Streams in the Upper Mississippi River Basin Study
This table contains a taxonomic list of benthic invertebrates collected from streams in the Upper Mississippi River Basin study unit as part of the USGS National Water Quality Assessemnt (NAWQA) Program. Invertebrates were collected from woody snags in selected streams from 1996-2004. Data Retreival occurred 26-JAN-06 11.10.25 AM from the USGS data warehouse (Taxonomic List Invert http://water.usgs.gov/nawqa/data). The data warehouse currently contains invertebrate data through 09/30/2002. Invertebrate taxa can include provisional and conditional identifications. For more information about invertebrate sample processing and taxonomic standards see, "Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory -- Processing, taxonomy, and quality control of benthic macroinvertebrate samples", at << http://nwql.usgs.gov/Public/pubs/OFR00-212.html >>. Data Retrieval Precaution: Extreme caution must be exercised when comparing taxonomic lists generated using different search criteria. This is because the number of samples represented by each taxa list will vary depending on the geographic criteria selected for the retrievals. In addition, species lists retrieved at different times using the same criteria may differ because: (1) the taxonomic nomenclature (names) were updated, and/or (2) new samples containing new taxa may Phylum Class Order Family Subfamily Tribe Genus Species Taxon Porifera Porifera Cnidaria Hydrozoa Hydroida Hydridae Hydridae Cnidaria Hydrozoa Hydroida Hydridae Hydra Hydra sp. Platyhelminthes Turbellaria Turbellaria Nematoda Nematoda Bryozoa Bryozoa Mollusca Gastropoda Gastropoda Mollusca Gastropoda Mesogastropoda Mesogastropoda Mollusca Gastropoda Mesogastropoda Viviparidae Campeloma Campeloma sp. Mollusca Gastropoda Mesogastropoda Viviparidae Viviparus Viviparus sp. Mollusca Gastropoda Mesogastropoda Hydrobiidae Hydrobiidae Mollusca Gastropoda Basommatophora Ancylidae Ancylidae Mollusca Gastropoda Basommatophora Ancylidae Ferrissia Ferrissia sp. -

Diptera: Chironomidae) from the Czech Republic
ISSN 2336 - 3193 Acta Mus. Siles. Sci. Natur., 65: 143 - 147 , 2016 DOI: 10.1515/cszma - 2016 - 001 8 P ublished : online August 201 6, in print 1 st September 2016 Some new records of chironomids (Diptera: Chironomidae) from the Czech Republic Peter Bitušík & Katarína Trnková Some new records of chironomids (Diptera: Chironomidae) from the Czech Republic. – Acta Mus. Siles. Sci. Natur. 65: 143 - 1 47 , 2016. Abstract : Six chironomid species: Paraboreochlus minutissimus (Strobl, 1894), Trissopelopia longimanus (Staeger 1839), Boreoheptagyia monticola (Serra - Tosio, 1964), Cricotopus (s.str.) similis Goetghebuer 1921, Heleniella serratosioi Ringe, 1976, Krenosmittia camptophleps (Edwards, 1929), were recorded in Czech Republic for the first time . The pupal exuviae were collected in July 2009 from Otava River in the vicinity of Rejštejn village in the central part of the Bohemian Forest. The notes on known distribution and ecology of the species are presented. Key words : Chironomidae, Podonominae, Tanypodinae, Diamesinae, Orthocl adiinae, ecological notes, distribution Introduction Pupal exuviae sampling has proved to be an excellent tool to identify the occurrence, distribution and ecology of chironomids in various water biotopes. This method greatly contributed, among other things, to the knowledge of the regional fauna (see e.g. B itušík 1993). Last time, Syrovátka & Langton (2015) demonstrated again that even one sample taken from a locality can bring surprising results and this paper also proves it. During the limnological investigations of the lakes in the Bohemian Forest, one sa mple of pupal exuviae was taken from Otava River in the vicinity of Rejštejn village in July 28, 2009. Here we present the results of our survey. -

Onchidoris Bilamellata Class: Gastropoda, Heterobranchia, Euthyneura, Ringipleura
Phylum: Mollusca Onchidoris bilamellata Class: Gastropoda, Heterobranchia, Euthyneura, Ringipleura Order: Nudipleura, Nudibranchia, Doridina, Doridoidei Many-gilled onchidoris nudibranch Family: Onchidoridoidea, Onchidorididae Description the dorid, with a variable number of lateral Size: Usual length 15 mm (McDonald 1980); pinnules. Proximal pinnules are larger than this specimen 15.5 mm long, 11 mm wide, 6 distal (Potts 1981). Unipinnate. Simple pin- mm high. Far northern and Atlantic speci- nate gills form almost erect branchial plumes mens can reach 31 mm length (Marcus arranged in two semicircles just anterior to the 1961). anus. Gills are not completely retractable Color: Translucent brownish-white with ir- (Kozloff 1974) (Fig. 1). While there is no bran- regular dark or rusty brown splotches, some- chial pocket into which the gills can be with- times as irregular longitudinal stripes. Com- drawn, there are localized muscle fibers monly a light spot between the dark rhino- around the base of each gill; contraction caus- phores; gills dull white, underside a dull es a depression in the mantle surface, and white (Marcus 1961). No yellow pigment, but gills can be brought in closer to the body some specimens without brown color (Potts 1981). Countercurrent exchange (Kozloff 1974). Cryptic coloration (Potts through the gills (Potts 1981). Clusters of 1981). branchial glands between every two gills Body: Doridiform: oval; slightly broadened (Marcus 1961). towards front. With a broad flat foot, thick Eggs: Type A, defined as an egg mass in rib- fleshy mantle, and conspicuous double cir- bon form, attached along the length of one clet of gills dorsally (Figs. 1, 2). Dorsum cov- edge, with capsules occurring throughout ered with many large round papillae, becom- (Hurst 1967). -

Phylogenomic Analysis and Morphological Data Suggest Left-Right Swimming Behavior Evolved Prior to the Origin of the Pelagic Phylliroidae (Gastropoda: Nudibranchia)
Phylogenomic analysis and morphological data suggest left-right swimming behavior evolved prior to the origin of the pelagic Phylliroidae (Gastropoda: Nudibranchia) Jessica A. Goodheart & Heike Wägele Organisms Diversity & Evolution ISSN 1439-6092 Org Divers Evol DOI 10.1007/s13127-020-00458-9 1 23 Your article is protected by copyright and all rights are held exclusively by Gesellschaft für Biologische Systematik. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Organisms Diversity & Evolution https://doi.org/10.1007/s13127-020-00458-9 ORIGINAL ARTICLE Phylogenomic analysis and morphological data suggest left-right swimming behavior evolved prior to the origin of the pelagic Phylliroidae (Gastropoda: Nudibranchia) Jessica A. Goodheart1 & Heike Wägele2 Received: 13 March 2020 /Accepted: 1 September 2020 # Gesellschaft für Biologische Systematik 2020 Abstract Evolutionary transitions from benthic to pelagic habitats are major adaptive shifts. Investigations into such shifts are critical for understanding the complex interaction between co-opting existing traits for new functions and novel traits that originate during or post-transition.