Colonial Breeding in the Barn Swallow (Hirundo Rustica) and Its Adaptive Significance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Nestling Diet on Growth and Adult Size of Zebra Finches (Poephila Guttata )
THE AUK A QUARTERLY JOURNAL OF ORNITHOLOGY VOL. 104 APRIL 1987 NO. 2 EFFECTS OF NESTLING DIET ON GROWTH AND ADULT SIZE OF ZEBRA FINCHES (POEPHILA GUTTATA ) PETER T. BOAG Departmentof Biology,Queen's University, Kingston, Ontario K7L 3N6, Canada Al•STRACT.--Manipulationof the diet of Zebra Finch (Poephilaguttata) nestlings in the laboratoryshowed that a low-quality diet reducedgrowth ratesof nine externalmorpholog- ical characters,while a high-quality diet increasedgrowth rates.The growth of plumage characterswas least affectedby diet, while growth ratesof tarsusand masswere most af- fected. The treatments also produced differencesin the adult size of experimental birds, differencesnot evident in either their parentsor their own offspring.Diet quality had the strongestimpact on adult massand tarsuslength, while plumage and beak measurements were less affected. Analysis using principal componentsand characterratios showed that the shapeof experimentalbirds was affectedby the experimentaldiets, but to a minor extent comparedwith changesin overall size. Significantshape changes involved ratiosbetween fast- and slow-growingcharacters. The ratios of charactersthat grow at similar, slow rates (e.g. beak shape) were not affected by the diets. Environmental sourcesof morphological variation should not be neglectedin studiesof phenotypicvariation in birds. Received5 June 1986, accepted30 October1986. MORPHOLOGICAL differences between indi- fitness, and weather was seen in the nonran- vidual birds are often assignedfunctional sig- dom survival of House Sparrows collected by nificance, whether those individuals are of dif- Hiram Bumpus following a winter storm ferent species,different sexes,or different-size (O'Donald 1973, Fleischer and Johnston 1982). members of the same sex (Hamilton 1961, Se- Recently, investigators have tried to dem- lander 1966,Clark 1979,James 1982). -

Cali Types of the Common Swift Apus Apus: Aduit Cali Given at the Nest
Avocetta W 17: 141-146 (1993) Cali types of the Common Swift Apus apus: aduIt cali given at the nest VrNCENT BRETAGNOLLE CEBC-CNRS,F-79360-Beauvoirsur Niort, France Abstract - Vocalizations of the Common Swifts were studi ed during two consecutive springs in southern France. I found that three cali types were given by the adults at the nest, and these are described quantitatively. Significant differences in the acoustic parameters of the calls are highlighted, as well as. a probable sexual dimorphism. This, together with the precise signification of the different cali types, rernam however to be critically assessed by playback experiments. Introduction France), where a large colony is easily accessible for field studies (for further details, see Gory, this Swifts (Order Apodiformes) are unusual among birds volume).A sample of 22 accessible nests was because many of their Iife history traits (i.e. longevity, selected, for which extensive data on breeding biology delayed maturity, large egg size) make them atypical and breeding success were also available dating from compared to similar-sized birds (Lack 1956, Gaillard 1978 (Gory et Jeantet 1986). The birds were tape et al. 1989), and convergent evolution of life histories recorded by Uher 4400 or Nagra IV at a speed of 19.5 with, e.g., long lived seabirds such as cmJs, on Agfa PE43 tapes, and with a Seinnheiser Procellariiformes, have been suggested repeatedly omnidirectional microphone MD421 placed within, or (e.g. Lack and Lack 1951, Malacarne et al. 1991). at the entrance of, the nesting cavity. Calls were Although investigations have been carried out on the analysed on a Real Time Spectrograph, which Common Swift Apus apus with regard to demography performs a fast Fourier transform (Richard 1991) at a (Lack 1956, Perrins 1971, Lebreton et al. -

Barn Swallows AKA: Mud Swallows
Barn Swallows AKA: Mud Swallows. Close relatives: Purple Martin, Cliff Swallow, Tree Swallow Scientific Classification: Animalia, Chordata, Aves, Passeriformes, Hirundinidae; Hirundo; H. rustica. Bird Size & Markings: Adult Barn Swallows are about 7” long, stand 4” high and have a 13” wingspan. They weigh less than 1 ounce. Males have metallic blue back, wings, and tail with rufous to tawny underside. The blue crown and face is contrasted with the cinnamon forehead and throat. Females are not as brightly colored. Habitat: You can find Barn Swallows feeding in open habitats such as fields, parks, marshes, meadows, ponds, and coastal waters. Their nests are often easy to spot under protected overhangs. Nesting/Dens: Barn Swallows lay 3 to 7 eggs in each brood and can hatch broods twice a year. Brood fledge in about 2 weeks. Both sexes construct the nest of mud pel- lets. If attached to a wall or beam, the nest is half-cup shaped. If on top of a surface, A mating pair of Barn Swallows. They prefer to the nest forms a perfect small cup about 3” wide. Nest sites are almost exclusively at- build their nests where there is overhead pro- tached to man made structures with overhead protection; roof eaves, the underside tection from the weather. of bridges, inside barns and stables, etc. Food: Barn Swallows eat insects - both flying and terrestrial. They usually take rela- tively large, single insects rather than feeding on swarms of smaller prey. They typi- cally feed just above shallow waters or turf. They have been known to follow tractors and livestock, eating the insects that are flushed out by their movement. -

Tree Swallows (Tachycineta Bicolor) Nesting on Wetlands Impacted by Oil Sands Mining Are Highly Parasitized by the Bird Blow Fly Protocalliphora Spp
Journal of Wildlife Diseases, 43(2), 2007, pp. 167–178 # Wildlife Disease Association 2007 TREE SWALLOWS (TACHYCINETA BICOLOR) NESTING ON WETLANDS IMPACTED BY OIL SANDS MINING ARE HIGHLY PARASITIZED BY THE BIRD BLOW FLY PROTOCALLIPHORA SPP. Marie-Line Gentes,1 Terry L. Whitworth,2 Cheryl Waldner,3 Heather Fenton,1 and Judit E. Smits1,4 1 Department of Veterinary Pathology, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B4, Canada 2 Whitworth Pest Solutions, Inc., 2533 Inter Avenue, Puyallup, Washington, USA 3 Department of Large Animal Clinical Sciences, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B4, Canada 4 Corresponding author (email: [email protected]) ABSTRACT: Oil sands mining is steadily expanding in Alberta, Canada. Major companies are planning reclamation strategies for mine tailings, in which wetlands will be used for the bioremediation of water and sediments contaminated with polycyclic aromatic hydrocarbons and naphthenic acids during the extraction process. A series of experimental wetlands were built on companies’ leases to assess the feasibility of this approach, and tree swallows (Tachycineta bicolor) were designated as upper trophic biological sentinels. From May to July 2004, prevalence and intensity of infestation with bird blow flies Protocalliphora spp. (Diptera: Calliphoridae) were measured in nests on oil sands reclaimed wetlands and compared with those on a reference site. Nestling growth and survival also were monitored. Prevalence of infestation was surprisingly high for a small cavity nester; 100% of the 38 nests examined were infested. Nests on wetlands containing oil sands waste materials harbored on average from 60% to 72% more blow fly larvae than those on the reference site. -

Aves: Hirundinidae)
1 2 Received Date : 19-Jun-2016 3 Revised Date : 14-Oct-2016 4 Accepted Date : 19-Oct-2016 5 Article type : Original Research 6 7 8 Convergent evolution in social swallows (Aves: Hirundinidae) 9 Running Title: Social swallows are morphologically convergent 10 Authors: Allison E. Johnson1*, Jonathan S. Mitchell2, Mary Bomberger Brown3 11 Affiliations: 12 1Department of Ecology and Evolution, University of Chicago 13 2Department of Ecology and Evolutionary Biology, University of Michigan 14 3 School of Natural Resources, University of Nebraska 15 Contact: 16 Allison E. Johnson*, Department of Ecology and Evolution, University of Chicago, 1101 E 57th Street, 17 Chicago, IL 60637, phone: 773-702-3070, email: [email protected] 18 Jonathan S. Mitchell, Department of Ecology and Evolutionary Biology, University of Michigan, 19 Ruthven Museums Building, Ann Arbor, MI 48109, email: [email protected] 20 Mary Bomberger Brown, School of Natural Resources, University of Nebraska, Hardin Hall, 3310 21 Holdrege Street, Lincoln, NE 68583, phone: 402-472-8878, email: [email protected] 22 23 *Corresponding author. 24 Data archiving: Social and morphological data and R code utilized for data analysis have been 25 submitted as supplementary material associated with this manuscript. 26 27 Abstract: BehavioralAuthor Manuscript shifts can initiate morphological evolution by pushing lineages into new adaptive 28 zones. This has primarily been examined in ecological behaviors, such as foraging, but social behaviors 29 may also alter morphology. Swallows and martins (Hirundinidae) are aerial insectivores that exhibit a This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. -

Birds Along Lehi's Trail
Journal of Book of Mormon Studies Volume 15 Number 2 Article 10 7-31-2006 Birds Along Lehi's Trail Stephen L. Carr Follow this and additional works at: https://scholarsarchive.byu.edu/jbms BYU ScholarsArchive Citation Carr, Stephen L. (2006) "Birds Along Lehi's Trail," Journal of Book of Mormon Studies: Vol. 15 : No. 2 , Article 10. Available at: https://scholarsarchive.byu.edu/jbms/vol15/iss2/10 This Feature Article is brought to you for free and open access by the Journals at BYU ScholarsArchive. It has been accepted for inclusion in Journal of Book of Mormon Studies by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Title Birds Along Lehi’s Trail Author(s) Stephen L. Carr Reference Journal of Book of Mormon Studies 15/2 (2006): 84–93, 125–26. ISSN 1065-9366 (print), 2168-3158 (online) Abstract When Carr traveled to the Middle East, he observed the local birds. In this article, he suggests the possi- bility that the Book of Mormon prophet Lehi and his family relied on birds for food and for locating water. Carr discusses the various birds that Lehi’s family may have seen on their journey and the Mosaic law per- taining to those birds. Birds - ALOnG LEHI’S TRAIL stephen l. cARR 84 VOLUME 15, NUMBER 2, 2006 PHOTOGRAPHy By RICHARD wELLINGTOn he opportunity to observe The King James translators apparently ex- birds of the Middle East came to perienced difficulty in knowing exactly which me in September 2000 as a member Middle Eastern birds were meant in certain pas- Tof a small group of Latter-day Saints1 traveling in sages of the Hebrew Bible. -

The Evolution of Nest Construction in Swallows (Hirundinidae) Is Associated with the Decrease of Clutch Size
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Linzer biol. Beitr. 38/1 711-716 21.7.2006 The evolution of nest construction in swallows (Hirundinidae) is associated with the decrease of clutch size P. HENEBERG A b s t r a c t : Variability of the nest construction in swallows (Hirundinidae) is more diverse than in other families of oscine birds. I compared the nest-building behaviour with pooled data of clutch size and overall hatching success for 20 species of swallows. The clutch size was significantly higher in temperate cavity-adopting swallow species than in species using other nesting modes including species breeding in evolutionarily advanced mud nests (P<0.05) except of the burrow-excavating Bank Swallow. Decrease of the clutch size during the evolution of nest construction is not compensated by the increase of the overall hatching success. K e y w o r d s : Hirundinidae, nest construction, clutch size, evolution Birds use distinct methods to avoid nest-predation: active nest defence, nest camouflage and concealment or sheltered nesting. While large and powerful species prefer active nest-defence, swallows and martins usually prefer construction of sheltered nests (LLOYD 2004). The nests of swallows vary from natural cavities in trees and rocks, to self-exca- vated burrows to mud retorts and cups attached to vertical faces. Much attention has been devoted to the importance of controlling for phylogeny in com- parative tests (HARVEY & PAGEL 1991), including molecular phylogenetic studies of swallows (WINKLER & SHELDON 1993). Interactions between the nest-construction va- riability and the clutch size, however, had been ignored. -

Head-Scratching Method in Swallows Depends on Behavioral Context
SHORT COMMUNICATIONS 679 shoulder-spot display during their observations of behavior in partridges. In all cases that I observed, the shoulder spot appeared to be a fear or flight intention display as described by Lumsden (1970). However, the display seemed secondary in importance compared to vocalizations and “tail flicking” during periods of extreme alarm. Examination of the shoul- der spot of a partridge confirmed the realignment of white underwing coverts to the top of the wing in the patagial region. The manipulation by the bird of underwing feathers appeared to be identical to that of Ruffed Grouse (Bonusa umbellus)(Garbutt 198 1). Since “display” implies actual communication between individuals further investigation is needed to de- termine if, in fact, the shoulder spot actually is serving a communication function in Gray Partridge. The shoulder spot in Gray Partridges and the display seen in grouse are morphologically similar. Lumsden (1970) concluded that the widespread occurrence of this display among grouse indicated it appeared relatively early in evolution. The morphological and behavioral similarities between the display in grouse and partridges suggest that the shoulder spot may have evolved even earlier. Since this is an escape behavior, and since many species of partridges and pheasants are difficult to observe in the wild, it may have been overlooked. Acknowledgments.-Theseobservations were made while the author was supported by funds from the North Dakota Game and Fish Department through Pittman-Robertson Project W-67-R. Additional support was provided by the Biology Department and Institute for Ecological Studies at the University of North Dakota. Helpful editorial comments were provided by R. -

Blue Swallow Survey Report November
Blue Swallow Survey Report November 2013- March 2014 By Fadzai Matsvimbo (BirdLife Zimbabwe) with assistance from Tendai Wachi ( Zimbabwe Parks and Wildlife Management Authority) Background The Blue Swallow Hirundo atrocaerulea is one of Africa’s endemics, migrating between East and Central to Southern Africa where it breeds in the summer. These breeding grounds are in Zimbabwe, South Africa, Swaziland, Mozambique, Malawi, southern Tanzania and south eastern Zaire, Zambia. The bird winters in northern Uganda, north eastern Zaire and Western Kenya (Keith et al 1992).These intra-african migrants arrive the first week of September and depart in April In Zimbabwe (Snell 1963.).There are reports of the birds returning to their wintering grounds in May (Tree 1990). In Zimbabwe, the birds are restricted to the Eastern Highlands where they occur in the Afromontane grasslands. The Blue Swallow is distributed from Nyanga Highlands southwards through to Chimanimani Mountains and are known to breed from 1500m - 2200m (Irwin 1981). Montane grassland with streams forming shallow valleys and the streams periodically disappearing underground and forming shallow valleys is the preferred habitat (Snell 1979). Whilst birds have been have only ever been located in the Eastern Highlands there is a solitary record from then Salisbury now Harare (Brooke 1962). The Blue Swallow is a medium sized swallow of about 20- 25 cm in body length. The males and females can be told apart by the presence of the long tail retrices in the male. The tail streamers in the males measure twice as long as the females (Maclean 1993). The adults are a shiny blue-black with a black tail with blue green gloss and whitish feather shafts. -

Birding Tour to Ghana Specializing on Upper Guinea Forest 12–26 January 2018
Birding Tour to Ghana Specializing on Upper Guinea Forest 12–26 January 2018 Chocolate-backed Kingfisher, Ankasa Resource Reserve (Dan Casey photo) Participants: Jim Brown (Missoula, MT) Dan Casey (Billings and Somers, MT) Steve Feiner (Portland, OR) Bob & Carolyn Jones (Billings, MT) Diane Kook (Bend, OR) Judy Meredith (Bend, OR) Leaders: Paul Mensah, Jackson Owusu, & Jeff Marks Prepared by Jeff Marks Executive Director, Montana Bird Advocacy Birding Ghana, Montana Bird Advocacy, January 2018, Page 1 Tour Summary Our trip spanned latitudes from about 5° to 9.5°N and longitudes from about 3°W to the prime meridian. Weather was characterized by high cloud cover and haze, in part from Harmattan winds that blow from the northeast and carry particulates from the Sahara Desert. Temperatures were relatively pleasant as a result, and precipitation was almost nonexistent. Everyone stayed healthy, the AC on the bus functioned perfectly, the tropical fruits (i.e., bananas, mangos, papayas, and pineapples) that Paul and Jackson obtained from roadside sellers were exquisite and perfectly ripe, the meals and lodgings were passable, and the jokes from Jeff tolerable, for the most part. We detected 380 species of birds, including some that were heard but not seen. We did especially well with kingfishers, bee-eaters, greenbuls, and sunbirds. We observed 28 species of diurnal raptors, which is not a large number for this part of the world, but everyone was happy with the wonderful looks we obtained of species such as African Harrier-Hawk, African Cuckoo-Hawk, Hooded Vulture, White-headed Vulture, Bat Hawk (pair at nest!), Long-tailed Hawk, Red-chested Goshawk, Grasshopper Buzzard, African Hobby, and Lanner Falcon. -
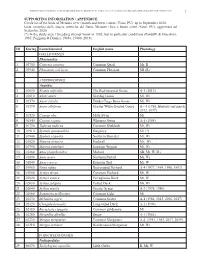
1 ID Euring Latin Binomial English Name Phenology Galliformes
BIRDS OF METAURO RIVER: A GREAT ORNITHOLOGICAL DIVERSITY IN A SMALL ITALIAN URBANIZING BIOTOPE, REQUIRING GREATER PROTECTION 1 SUPPORTING INFORMATION / APPENDICE Check list of the birds of Metauro river (mouth and lower course / Fano, PU), up to September 2020. Lista completa delle specie ornitiche del fiume Metauro (foce e basso corso /Fano, PU), aggiornata ad Settembre 2020. (*) In the study area 1 breeding attempt know in 1985, but in particolar conditions (Pandolfi & Giacchini, 1985; Poggiani & Dionisi, 1988a, 1988b, 2019). ID Euring Latin binomial English name Phenology GALLIFORMES Phasianidae 1 03700 Coturnix coturnix Common Quail Mr, B 2 03940 Phasianus colchicus Common Pheasant SB (R) ANSERIFORMES Anatidae 3 01690 Branta ruficollis The Red-breasted Goose A-1 (2012) 4 01610 Anser anser Greylag Goose Mi, Wi 5 01570 Anser fabalis Tundra/Taiga Bean Goose Mi, Wi 6 01590 Anser albifrons Greater White-fronted Goose A – 4 (1986, february and march 2012, 2017) 7 01520 Cygnus olor Mute Swan Mi 8 01540 Cygnus cygnus Whooper Swan A-1 (1984) 9 01730 Tadorna tadorna Common Shelduck Mr, Wi 10 01910 Spatula querquedula Garganey Mr (*) 11 01940 Spatula clypeata Northern Shoveler Mr, Wi 12 01820 Mareca strepera Gadwall Mr, Wi 13 01790 Mareca penelope Eurasian Wigeon Mr, Wi 14 01860 Anas platyrhynchos Mallard SB, Mr, W (R) 15 01890 Anas acuta Northern Pintail Mi, Wi 16 01840 Anas crecca Eurasian Teal Mr, W 17 01960 Netta rufina Red-crested Pochard A-4 (1977, 1994, 1996, 1997) 18 01980 Aythya ferina Common Pochard Mr, W 19 02020 Aythya nyroca Ferruginous -

Fastest Migration Highest
GO!” Everyone knows birds can fly. ET’S But not everyone knows that “L certain birds are really, really good at it. Meet a few of these champions of the skies. Flying Acby Ellen eLambeth; art sby Dave Clegg! Highest You don’t have to be a lightweight to fly high. Just look at a Ruppell’s griffon vulture (left). One was recorded flying at an altitude of 36,000 feet. That’s as high as passenger planes fly! In fact, it’s so high that you would pass out from lack of oxygen if you weren’t inside a plane. How does the vulture manage? It has Fastest (on the level) Swifts are birds that have that name for good special blood cells that make a small amount reason: They’re speedy! The swiftest bird using its own of oxygen go a long way. flapping-wing power is the common swift of Europe, Asia, and Africa (below). It’s been clocked at nearly 70 miles per hour. That’s the speed limit for cars on some highways. Vroom-vroom! Fastest (in a dive) Fastest Migration With gravity helping out, a bird can pick up extra speed. Imagine taking a trip of about 4,200 And no bird can go faster than a peregrine falcon in a dive miles. Sure, you could easily do it in an airplane. after prey (right). In fact, no other animal on Earth can go as But a great snipe (right) did it on the wing in just fast as a peregrine: more than 200 miles per hour! three and a half days! That means it averaged about 60 miles The prey, by the way, is usually another bird, per hour during its migration between northern which the peregrine strikes in mid-air with its balled-up Europe and central Africa.