Clinio-Pathological Changes in Goats Challenged with Corynebacterium Peudotuberculosis and Its Exotoxin (PLD)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antibodies to Corynebacterium Pseudotuberculosis in Adult Goats from a Naturally Infected Herd1\"
Acta vet. scand. 1982, 23. 473-482. From the Department of Microbiology and Immunology, Norwegian College of Veterinary Medicine, Oslo, and Department of Animal Gene tics and Breeding, Agricultural University of Norway, As. ANTIBODIES TO CORYNEBACTERIUM PSEUDOTUBERCULOSIS IN ADULT GOATS FROM A NATURALLY INFECTED HERD1\" By Arve Lund, Torbjern Almlid, Hans Larsen and Torstein Steine LUND, ARVE, TORBJ0RN ALMLID, HANS J0RGEN LARSEN and TORSTEIN STEINE: Antibodies to Corynebacterium pseudotubercu losis in adult goats from a naturally infected herd. Acta vet. scand. 1982,23,473-482.- Serum samples taken in 3 successive years (1977, 1978 and 1979) from adult dairy goats (Norwegian breed) or-iginating from 1 herd were examined for antibodies to Corynebacterium pseu dotuberculosis. Both bacterial agglutination test (BAT) and hemolysis inhibition test (HIT) were used. The llroportion of seropositive goats increased 10-12 % during the Investtgation period. In 1979 all ani mals were seropositive to BAT and about 95 % had antihemolysins in their sera. Twenty-two of the 23 one-year old goats recruited to the herd in 1978 were seropositive. The average age-specific titres in creased up to the age of 3 years, and subsequently decreased for goats aged 4-7 years. Caseous lymphadenitis is thus regarded as a chronic infection. The effect of age on the titre values was significant at the 5 % level in 1977 and 1978 when HIT was used and in 1978 when BAT was used. During the investigation period the same 36 and goats were examined every year by BAT and HIT, respectively. Intermediate to high correlations between titre values for the same goats from year to year were found. -

Capra Pyrenaica
Colom-Cadena et al. Acta Veterinaria Scandinavica 2014, 56:83 http://www.actavetscand.com/content/56/1/83 RESEARCH Open Access Management of a caseous lymphadenitis outbreak in a new Iberian ibex (Capra pyrenaica) stock reservoir Andreu Colom-Cadena1, Roser Velarde1, Jes?s Salinas 2, Carmen Borge3, Ignacio Garc?a-Bocanegra 3, Emmanuel Serrano1,4, Diana Gass? 1, Ester Bach1, Encarna Casas-D?az 1, Jorge R L?pez-Olvera 1, Santiago Lav?n 1, Lu?s Le?n-Vizca?no 2 and Gregorio Mentaberre1* Abstract Background: In 2010, an Iberian ibex (Capra pyrenaica hispanica) stock reservoir was established for conservation purposes in north-eastern Spain. Eighteen ibexes were captured in the wild and housed in a 17 hectare enclosure. Once in captivity, a caseous lymphadenitis (CLA) outbreak occurred and ibex handlings were carried out at six-month intervals between 2010 and 2013 to perform health examinations and sampling. Treatment with a bacterin-based autovaccine and penicillin G benzatine was added during the third and subsequent handlings, when infection by Corynebacterium pseudotuberculosis was confirmed. Changes in lesion score, serum anti-C. pseudotuberculosis antibodies and haematological parameters were analyzed to assess captivity effects, disease emergence and treatment efficacy. Serum acute phase proteins (APP) Haptoglobin (Hp), Amyloid A (SAA) and Acid Soluble Glycoprotein (ASG) concentrations were also determined to evaluate their usefulnessasindicatorsofclinical status. Once in captivity, 12 out of 14 ibexes (85.7%) seroconverted, preceding the emergence of clinical signs; moreover, TP, WBC, eosinophil and platelet cell counts increased while monocyte and basophil cell counts decreased. After treatment, casualties and fistulas disappeared and both packed cell volume (PCV) and haemoglobin concentration significantly increased. -

Caseous Lymphadenitis (Cheesy Gland)
Goat health - caseous lymphadenitis (cheesy gland or CLA) August 2017, Primefact 1595 Animal Biosecurity and Welfare, NSW DPI Caseous lymphadenitis (CLA) is a recurring bacterial disease in goats that causes abscesses in lymph nodes in internal organs and under the skin. It is the cause of extensive loss through carcase condemnation in sheep and, as the goat meat industry increases, a similar substantial loss is likely in the goat industry. Cause Cheesy gland is caused by infection with the bacterium Corynebacterium pseudotuberculosis. The organism occurs in abscesses as well as in the gut and faeces of the goat. It can survive for up to four months on the ground and on fences, feed troughs and head bails depending on shelter from wind and sun. Spread of infection The bacteria are abundant in the pus inside abscesses. When these abscesses burst, the pus containing bacteria is transferred to the environment around the goat pens. The infection is then picked up by other goats through contamination of wounds and broken skin. The common behavioural habit of frequent licking, as well as rubbing their heads and necks against fence posts and sheds, allows the rapid spread of cheesy gland. Where goats are kept in small yards, the direct contact and close grazing of contaminated grass or food in feed troughs encourages spread. Dairy goats that are placed in head bails for milking are particularly prone to being infected through splinters around the neck. Contaminated grooming gear can spread the bacteria to other goats. Contaminated shearing blades are an important method of spread in Angoras and Cashmere goats. -

Caseous Lymphadenitis (CLA) in Sheep and Goats
Caseous lymphadenitis (CLA) in sheep and goats BVA Congress, Belfast Frank Malone Veterinary Sciences Division Agri-Food and Biosciences Institute CLA – presentation outline • Introducing CLA • Control of disease • Prevalence of disease • Diagnosis • Flock eradication by serology • CLA study in 4 culled flocks Caseous Lymphadenitis (CLA) • Corynebacterium pseudotuberculosis • First diagnosed in GB sheep in 1991 (goats 1990) – NI in 1999 – RoI in 1998 • Mainly terminal sire breeds • Prevalence in Britain may be as high as 18% of flocks • Prevalence of > 25% within some affected flocks CLA infection • Infection enters through wounds – Abscesses in lymph nodes – Lung and viscera abscesses • Spreads rapidly within flock •Losses – Culling affected sheep – Affected parts of carcase condemned • downgrading of carcase – Trade implications • Human infection - uncommon CLA in goats • Natural infection similar to sheep • Same biotype of C. pseudotuberculosis • Clinical presentation – Superficial lymph nodes • Mainly head and neck • Main route of infection – via oral cavity – Skin abrasions face and head • Visceral lesions more common in sheep CLA in Superficial Lymph Nodes Parotid Retropharyngeal Submandibular Popliteal Prescapular Prefemoral CLA – lymphatic drainage CLA in Parotid Lymph Node CLA in Parotid Lymph Node CLA in Parotid Lymph Node CLA abscesses in LNs CLA Abscesses in Lungs Differential diagnosis • Arcanobacterium pyogenes • Actinobacillus lignieresi (cruels) • Staphlococcal dermatitis CLA - problems with control • Carrier animals – -
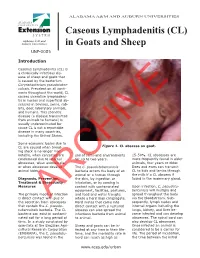
Caseous Lymphadenitis (CL) in Goats and Sheep UNP-0085
ALABAMA A&M AND AUBURN UNIVERSITIES Caseous Lymphadenitis (CL) in Goats and Sheep UNP-0085 Introduction Caseous Lymphadenitis (CL) is a chronically infectious dis- ease of sheep and goats that is caused by the bacterium Corynebacterium pseudotuber- culosis. Prevalent on all conti- nents throughout the world, CL causes ulcerative lymphadeni- tis in horses and superficial ab- scesses in bovines, swine, rab- bits, deer, laboratory animals, and humans. This zoonotic disease (a disease transmitted from animals to humans) is usually underestimated be- cause CL is not a reportable disease in many countries, including the United States. Some economic losses due to CL are caused when breed- Figure 1. CL abscess on goat. ing stock is no longer mar- ketable, when carcasses are soil of semi-arid environments 15-50%. CL abscesses are condemned due to internal for up to two years. more frequently found in older abscesses, when animals die, animals, four years or older. or when abscesses devalue The C. pseudotuberculosis Does and ewes can transmit animal hides. bacteria enters the body of an CL to kids and lambs through animal or a human through the milk if a CL abscess if Diagnosis, Prevention, the skin, by ingestion or found in the mammary gland. Treatment & Biosecurity inhalation, or by coming in Measures contact with contaminated Upon infection, C. pseudotu- equipment, facilities, pastures, berculosis will multiply and The primary mode of infection and feed and water troughs spread throughout the body is direct contact with pus or where a herd may congregate. via the bloodstream. Sub- the secretion from abscessesARCHIVE Herd mates that come into sequently, lymph nodes and that contain the C. -

Caseous Lymphadenitis in Small Ruminants
DIVISION OF AGRICULTURE RESEARCH & EXTENSION UJA--University of Arkansas System Agriculture and Natural Resources FSA3095 Livesto k Health Series Caseous Lymphadenitis in Small Ruminants The bacteria can survive for several months in the environment, so Introduction Caseous lymphadenitis (CL) is biosecurity protocols should be in Heidi Ward, a contagious disease of small rumi- place when treating and handling VM, Ph nants caused by the bacterium animals potentially infected by this Assistant Professor Corynebacterium pseudotuberculosis. organism. Disposable gloves and boot and Veterinarian The disease is found throughout the covers should always be worn during world and is a major concern for sheep inter action with suspect animals and and goat producers in the United hands, and clothes should always be Jeremy Powell, States as it causes economic loss from washed directly after contact. VM, Ph wool and hide loss, carcass condemna- Professor tion and death. CL is characterized by Clinical igns abscesses of subcutaneous lymph nodes (external form) and abscesses of The most obvious symptom of the disease is swelling corresponding with internal lymph nodes or organs (internal form). the abscessed lymph node just under the skin (external form). Sometimes, the bacteria can enter the bloodstream Transmission to cause abscesses in internal organs, Bacteria that cause CL enter such as the liver, lungs, kidney or through skin wounds or mucous reproductive tract, resulting in a thin membranes and then localize in and sickly animal with no other the lymph nodes to proliferate. Once obvious clinical signs (internal form). in the lymph nodes, the animal’s The lymph nodes around the head and natural immune defenses wall off neck region are most commonly the quickly-dividing bacteria, thus affected, but any lymph node in the forming an abscess. -

Serological Detection of Caseous Lymphadenitis in Sheep and Goats Using a Commercial ELISA in Grenada, West Indies
IBIMA Publishing International Journal of Veterinary Medicine: Research & Reports http://www.ibimapublishing.com/journals/IJVMR/ijvmr.html Vol. 2015 (2015), Article ID 473459, 7 pages DOI: 10.5171/2015.473459 Research Article Serological Detection of Caseous Lymphadenitis in Sheep and Goats Using a Commercial ELISA in Grenada, West Indies H. Hariharan 1 , K. P. Tiwari 2 , S. Kumthekar 3 , D. Thomas 4 , C. Hegamin-Younger 5 , B. Edwards 6 and R. N. Sharma 7 1,2,3,6,7 Department of Pathobiology, School of Veterinary Medicine, P.O. Box 7, St. George’s University, St. George, Grenada, West Indies 4 Ministry of Agriculture, Forestry and Fisheries, St. George, Grenada, West Indies 5 Department of Preventive Medicine and Public Health, School of Medicine, St. George’s University, St. George, Grenada, West Indies Correspondence should be addressed to H. Hariharan; [email protected] Received date: 22 May 2014; Accepted date: 30 August 2014; Published date: 5 October 2015 Academic Editor: Bhupendra Nath Tripathi Copyright © 2015. H. Hariharan, K. P. Tiwari , S. Kumthekar, D. Thomas , C. Hegamin-Younger, B. Edwards and R. N. Sharma. Distributed under Creative Commons CC-BY 4.0 Abstract Caseous lymphadenitis (CLA) in small ruminants has been diagnosed by culture several times in the state of Grenada. In order to understand the prevalence of CLA, serum samples from 541 sheep and 338 goats from Grenada and Carriacou islands were tested for antibodies against the phospholipase antigen of Corynebacterium pseudotuberculosis , the causative agent of caseous lymphadenitis (CLA) using a commercial ELISA kit: “ELITEST CLA”. The percentage of positive samples among sheep was 7.9, and for goats it was 31.3%, with a significant difference between these two species of animals. -

Mesenteric Caseous Lymphadenitis in a Cow Calf Caused by Corynebacterium Pseudotuberculosis: a Case Report
Veterinarni Medicina, 57, 2012 (7): 371–375 Case Report Mesenteric caseous lymphadenitis in a cow calf caused by Corynebacterium pseudotuberculosis: a case report N.K. Sood, B.S. Sandhu, K. Gupta, D. Narang, K. Vasudeva, N.D. Singh College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India ABSTRACT: Caseous lymphadenitis caused by Corynebacterium pseudotuberculosis is mainly a disease of sheep and goats and is of zoonotic importance. The disease has rarely been recorded in cattle and mostly in its superfi- cial form. The present communication deals with an extremely rare case of corynebacterium-induced mesenteric pseudotuberculosis in a cow calf. The gross, cytologic, histopathologic and microbial isolation as well as cultural characteristics of the organisms have been described, as well as the mode of spread of the disease to the mesenteric lymph nodes. To the authors’ knowledge, this is the first report of mesenteric caseous lymphadenitis in a cow calf. Keywords: caseous lymphadenitis; cow calf; Corynebacterium pseudotuberculosis; mesenteric; tape worms, CAMP Caseous lymphadenitis is a chronic contagious naturally (Shpigel et al. 1993), or experimentally disease of sheep, goat and occasionally cattle, deer, (Aroch et al. 2003). Yeruham et al (2003) reported horse, camelids, water buffalo, wild ruminants, pri- C. pseudotuberculosis infection in Israeli dairy cat- mates, pigs, and fowl. The disease is also of zoonotic tle, where the condition was manifested in cutane- importance as it may on rare occasions cause regional ous, mastitis and visceral forms. The isolation of lymphadenitis in humans, particularly in farm work- C. pseudotuberculosis from an ectopic site like the ers and meat inspectors (Peel et al. -

Caseous Lymphadenitis in Small Ruminants Dave Van Metre, DVM, DACVIM Professor / Extension Veterinarian Colorado State University
Caseous Lymphadenitis in Small Ruminants Dave Van Metre, DVM, DACVIM Professor / Extension Veterinarian Colorado State University Caseous lymphadenitis is a contagious bacterial infection of the lymph nodes of sheep and goats. It is caused by a bacterium called Corynebacterium pseudotuberculosis. This bacterium is extraordinarily durable, apparently able to survive in soil for months to years, even in dry climates with substantial sun exposure. It is commonly found in the soil on farms with infected animals. It causes the development of abscesses within the lymph nodes of infected sheep and goats. Abscesses can form as soon as 2 weeks after exposure to the organism, although it can take several months for abscesses to develop in some cases. Infection occurs through contact with pus containing the organism, which commonly occurs when an infected animal develops a draining wound emanating from an infected lymph node. The draining material may be directly transferred to another animal through contact, or it may contaminate the soil, bedding, water, or feed and be transferred through these routes. In dairy sheep and goats, contaminated milking machines can spread the organism. The organism can also be spread by shearing equipment, combs, or other tack that is contaminated with pus. Flies may spread the organism from one animal to another by feeding on infected material and moving to another animal to feed on a wound. There are two forms of the disease. In the external form, the lymph nodes immediately under the skin become infected and visibly enlarged, growing to a size of 1-2 inches to several inches in diameter. -

Innovative Resources in Small Ruminant Health Sarah Paluso [email protected]
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library Spring 5-10-2019 Innovative Resources in Small Ruminant Health Sarah Paluso [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Part of the Sheep and Goat Science Commons Recommended Citation Paluso, Sarah, "Innovative Resources in Small Ruminant Health" (2019). Electronic Theses and Dissertations. 2981. https://digitalcommons.library.umaine.edu/etd/2981 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. INNOVATIVE RESOURCES IN SMALL RUMINANT HEALTH By Sarah Paluso B.S. Gettysburg College, 2016 A THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Animal Science) The Graduate School The University of Maine May 2019 Advisory Committee: Anne Lichtenwalner, Associate Professor of Animal and Veterinary Sciences, Advisor Juan Romero, Assistant Professor of Animal Nutrition Con Sullivan, Assistant Professor of Biology Sally Molloy, Assistant Professor of Genomics INNOVATIVE RESOURCES IN SMALL RUMINANT HEALTH By Sarah Paluso Thesis Advisor: Dr. Anne Lichtenwalner An Abstract of the Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science (in Animal Science) May 2019 Caseous lymphadenitis (CL) is a chronic bacterial infection caused by Corynebacterium pseudotuberculosis (C. pseudoTB) that affects small ruminants. This disease has a worldwide prevalence and results in significant economic losses to the sheep and goat industries. -

CASEOUS LYMPHADENITIS in GOATS Introduction
Biotechnology in Animal Husbandry 25 (5-6), p 999-1007, 2009 ISSN 1450-9156 Publisher: Institute for Animal Husbandry, Belgrade-Zemun UDC 636.09 CASEOUS LYMPHADENITIS IN GOATS S. Ivanović1, M. Žutić1, I. Pavlović1, M. Žujović2 1Veterinary Institute of Serbia, 11000, Belgrade, Republic of Serbia 2Institute for Animal Husbandry, 11080, Belgrade-Zemun, Republic of Serbia Corresponding author: [email protected] Original scientific paper Abstract: Caseous lymphadenitis is a chronic disease of lymph nodes in goats and sheep, sometimes in humans. A cause is Corynebacterium pseudotuberculosis. The disease is manifested with development of abscesses, mostly in superficial lymph nodes. If disease is not generalized, general condition of animal does not need to be changed. This disease can influence big economic losses. Removing of muscle tissue with changed lymph nodes can cause damage of goat leather and its economic value could be quite diminished. If the disease is generalized it can cause death of adult animals and a breakdown of fertility. Changes on lymph nodes are noticed mostly during the slaughter. The aim of an inspection of goat carcasses was to provide hygienic safe meat (healthy) for human consumption. During the inspection of goats before and after slaughter, on the slaughter line we noticed an enlargement of prescapular and sub-mandibular lymph nodes on the right half (middle walnut size). The lymph nodes were forwarded to analysis in the laboratory. During the primary bacteriological treatment of samples it is established that both lymph nodes were hard, covered with connective tissue capsule with caseous content. In direct microscopic preparation colored by Gram, in these samples were found out Gram-positive bacilli. -

Major Zoonotic Diseases of Sheep and Meat Goats
SSERT-303 03-11 The Species Specific Educational fazd.tamu.edu Resource Team (SSERT) A Series for Small-Scale Producers and Hobby Owners Major Zoonotic Diseases of Sheep and Meat Goats Maria Lenira Leite-Browning, DVM, Alabama A&M University Cooperative Extension System; Richard Browning Jr., Ph.D., Associate Professor, Department of Agricultural Sciences, Tennessee State University; Cassandra F. Vaughn , D.V.M. Associate Professor, School of Agriculture, Alcorn State University Cooperative Extension Program; Kenneth Andries, Ph. D., Animal Science Specialist and Marion Simon, Ph. D., Small Farm Specialist, Kentucky State University Cooperative Extension Program Mrs. Wyvette Williams, Kentucky State University Cooperative Extension Program, Graphic Designer; Mr. Mitt Walker, Director of the Alabama Meat Goat and Sheep Commodity Group, Alabama Farmers Federation, Technical Input; Photograph complements of Kenneth Andries, Ph.D., Animal Science Specialist, Kentucky State University Keywords: Foreign Animal Diseases of extra-label use of products under veterinary supervision. Consult your veterinarian for disease prevention methods Goats and Sheep, Zoonotic Diseases of and needed vaccinations. Some diseases are ‘reportable’, Goats and Sheep meaning that they are regarded as high human or animal health risks by state and/or federal agencies. If a reportable oreign animal diseases are those that are not cur - disease is identified in an animal or herd, the case must be rently in the U.S. Zoonotic diseases are those that are reported to a state or federal animal health agency. Often Ftransferable between animals and humans. New your veterinarian does this. Many of these diseases are farming methods, international trade, and the influx of transmitted to humans by contaminated foods or by expo - people into rural areas and wildlife habitats contribute to sure to diseased animals.