Montserrat Is a Small Island in the Lesser Antilles, Approximately 30 Miles From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caribbean Food Crops Society
PROCEEDINGS OF THE CARIBBEAN FOOD CROPS SOCIETY EIGHTH ANNUAL MEETING SANTO DOMINGO DOMINICAN REPUBLIC 197 0 VOLUME VIII 29 THE EVALUATION OF TOMATO VARIETIES IN THE LEEWARD ISLANDS: A PROGRESS REPORT St. C. M. Forde Leeward Islands Agronomist University of the West Indies St. John's Antigua INTRODUCTION In the Leeward Islands the production of tomatoes is largely in the hands of peasant farmers who make use of commercially available varieties. The crop is established during the period September- November and matures in December-March which coincides with the dry season and also the period, of lowest mean minimum temperatures (70- 72°F). Even at the peak of production, local market demands in Antigua and St. Kitts are not satisfied, but production levels in Montserrat allow for some export of produce to the New York as well as other Caribbean markets. The main problems associated with the industry are the evalua tion of commercially available varieties according to time of plan- ting, and extending production into the dry season by the introduc- tion of irrigation. At the University of the West Indies research in tomato breeding is aimed at developing varieties for increased yield under dry season conditions and high yielding varieties that will set fruit at night temperatures above 72° for wet season production. However there is a pressing need in the Leeward Islands to examine the performance of the commercial varieties available in the area, especially in relation to time of planting. It is against this background that this work was undertaken. MATERIALS AND METHODS Five 6x5 randomised complete block trials were carried out in Antigua, Montserrat and St. -

Antigua and Barbuda Citizenship-By-Investment Programme: OPTION 1
INVESTMENT OPTIONS There are two investment options which serve as a qualifier for your ANTIGUA AND application to the Antigua and Barbuda citizenship-by-investment programme: OPTION 1 BARBUDA National Development Fund (NDF) Contribution Main applicant or a family of up to 4: USD 100,000. CITIZENSHIP BY INVESTMENT PROGRAMME For a family of 5 or more: USD 125,000. Global community - Global citizenship OPTION 2 Real Estate Investment ABOUT ANTIGUA AND BARBUDA Approved property: USD 200,000 or 400,000. Antigua and Barbuda is an independent Commonwealth state in the Eastern Caribbean. With GOVERNMENT FEES some 365 beaches of clean turquoise waters, the lush tropical islands of Antigua and Barbuda are an inviting paradise and considered to be one of the most beautiful places in the world. As a NDF: result, tourism is the key driver of Gross Domestic Product (GDP) and generates around 60% of Family up to 4: USD 25,000. the island’s income, with key target markets being the U.S., Canada and Europe. Antigua and Each additonal dependant: USD 15,000. Barbuda is a member of the United Nations, the British Commonwealth, CARICOM and the Real Estate: Organisation of American States (OAS) among many other international organisations. Family up to 4: USD 50,000. Each additional dependent: USD 15,000. WHY ANTIGUA AND BARBUDA TRAVEL WITH EASE PROCESS AND TIMELINE Month 1-2 Sign a retainer agreement and pay the first retainer invoice. The Antigua passport unlocks visa-free travel to over 140 countries including Hong Kong, Preparation Prepare required documentation. Singapore, the UK and the Schengen states.The passport is valid for a period of 5 years and will 10% payment of government fees. -

Antigua and Barbuda Bahamas Barbados Belize British Overseas Territories (Anguilla, Bermuda, British Virgin Islands, Cayman Isla
UNHCR staff monitoring programmes attheLoveAChild field hospital in Fond Parisien, Haiti. Antigua and Barbuda Bahamas Barbados Belize British overseas territories (Anguilla, Bermuda, British Virgin Islands, Cayman Islands, Turks and Caicos Islands, Montserrat) Canada Dominica Dominican Republic Dutch overseas territories in the Caribbean (Aruba, Curaçao, Saint Maarten, Bonaire, Saint Eustatius, Saba) French overseas departments (Martinique, Guadeloupe) Grenada Guyana Haiti Jamaica St. Kitts and Nevis St. Lucia St. Vincent and the Grenadines Suriname Trinidad and Tobago United States of America 348 UNHCR Global Report 2010 and the OPERATIONAL HIGHLIGHTS l UNHCR continued to seek the political and financial l More than 80 per cent of UNHCR’s global resettlement support of the Governments of the United States and referrals are to the United States and Canada. Canada in order to fulfil its protection mandate and find comprehensive solutions for refugees. Working environment l In the United States, UNHCR sought to ensure that the country’s laws and policies, as well as their implementation, In the United States, the Government has confirmed its were in accordance with its obligations under the 1967 commitment to international obligations, particularly with Protocol Relating to the Status of Refugees. Specifically, regard to the parole of asylum-seekers. However, UNHCR promoted reforms to the way in which the refugee adjudications by the immigration courts and administrative definition is being applied under US law and monitored the and federal -

The Post-Slavery Caribbean Plantation Complex∗
Franchise Extension and Elite Persistence: The Post-Slavery Caribbean Plantation Complex∗ Christian Dippely July 20th 2011 Preliminary - Comments Welcome Abstract What determines the extension of the franchise to the poor? This paper studies the 19th century development of parliamentary institutions in the 17 British Caribbean slave colonies. After abolition, freed slaves began to obtain the franchise as small- holders. I document that the elite’s response was a series of constitutional changes in which local parliaments voted to restrict their own powers or abolish themselves altogether in favor of direct colonial rule. This “defensive franchise contraction” hap- pened later or not at all where the franchise expanded less and political turnover re- mained lower, suggesting it was aimed at excluding the new smallholder class from the political process. Against its stated aim, direct colonial rule led to increases in coercive expenditure and decreases in public good provision, suggesting it did not reduce planter elites’ insider access to the colonial administration. A new data-set on the identity of all elected and nominated politicians in the colonies from 1838 to 1900 confirms that planter interests continued to dominate the reformed legislatures under direct colonial rule. Keywords: Economic Development, Elite Persistence, Political Inequality, Institutions, Fran- chise Extension. ∗I thank Toke Aidt, Dwayne Benjamin, Loren Brandt, Mauricio Drelichman, Gilles Duranton, Peter Morrow and Dan Trefler and participants of the 2011 CEGE conference for valuable discussions and insightful comments yDepartment of Economics, University of Toronto (email: [email protected]) 1 Introduction The 19th century is generally viewed as the century in which the franchise was for the first time extended to broad segments of society. -

Tier 1 Countries Annual Dues
Tier 1 Countries Tier 1 Countries Tier 2 Countries Tier 3 Countries Tier 4 Countries Annual Dues - $250 (continued) Annual Dues - $100 Annual Dues - $50 Annual Dues - $20 Qatar Andorra San Marino Albania Angola Afghanistan Antigua and Barbuda Saudi Arabia Algeria Anguilla Benin Argentina Seychelles American Samoa Bangladesh Burkina Faso Aruba Singapore Armenia Bhutan Burundi Australia Sint Maarten Azerbaijan Bolivia Central African Rep. Austria Slovak Republic Belarus Cabo Verde Chad Bahamas Slovenia Belize Cambodia Comoros Bahrain South Korea Bosnia/Herzegovina Cameroon Dem. Rep. of Congo Barbados Spain Botswana Congo Eritrea Belgium St Kitts/Nevis Brazil Cook Islands Ethiopia Bermuda St Martin Bulgaria Côte d’Ivoire Gambia British Virgin Islands Sweden China Djibouti Guinea Brunei Darussalam Switzerland Colombia Egypt Guinea-Bissau Canada Taiwan Costa Rica El Salvador Haiti Cayman Islands Trinidad/Tobago Cuba French Guiana Liberia Channel Islands Turks and Caicos Dominica French So. Territories Madagascar Chile United Arab Emirates Dominican Republic Georgia Malawi Croatia United Kingdom Ecuador Ghana Mali Curacao United States Equatorial Guinea Guadeloupe Mozambique Cyprus Uruguay Fiji Honduras Nepal Czech Republic Virgin Islands Gabon India Niger Denmark Grenada Indonesia North Korea Estonia Guatemala Kenya Rwanda Faroe Islands Guyana Kiribati Senegal Falkland Islands (Malvinas) Iran Kosovo Sierra Leon Finland Iraq Kyrgyz Republic Somalia France Jamaica Lao PDR South Sudan French Polynesia Jordan Lesotho Syrian Arab Republic Germany -

Citizenship, Descent and Place in the British Virgin Islands
Bill Maurer Sta1iford University Children of Mixed Marriages on Virgin Soil: Citizenship, Descent and Place in the British Virgin Islands In this essay, I describe a three-stranded argument over who has rightful claim to citizenship in the British Virgin Islands (BVI). Specifically, this essay demonstrates how the law conjures up a domain of "nature" that infonns positions in this argument. There are three parties to this debate: citizens of the BVI who never went abroad for education and employment, citizens of the BVI who left the territory in the 1960s and are now returning, and immigrants to the BVI and their children. For citizens who never emigrated, the law helps construct the nature of identity in tenns of descent, blood, and "race." For immigrants and their children, who make up fifty percent of the present population, the law lends emphasis to region and jurisdiction to construct identity in tenns of predetennined places. For return emigre British Virgin Islanders, the law enables notions of individual ability that appear to make place- and race-based identities superfluous. I will demonstrate how conceptions of belonging and identity based on place and conceptions based on descent work in concert with notions of individual ability to naturalize inequalities between British Virgin Islanders and immigrants. Specifically, I will focus on the inequalities between those who emigrated and those who stayed behind, and between those who emigrated and those BVIslander and immigrants who now work for them. I argue that the law, especially citizenship but also the laws of jurisdictional division, is critical to the creation and maintenance of these competing identities and the inequalities they cover over or remove from discussion. -
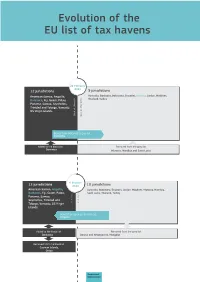
Evolution of the EU List of Tax Havens
Evolution of the EU list of tax havens 22 February 2021 12 jurisdictions 9 jurisdictions American Samoa, Anguilla, Australia, Barbados, Botswana, Eswatini, Jamaica, Jordan, Maldives, Dominica, Fiji, Guam, Palau, Thailand, Turkey Panama, Samoa, Seychelles, Trinidad and Tobago, Vanuatu, US Virgin Islands Moved from black list to grey list Barbados Added to the black list Removed from the grey list Dominica Morocco, Namibia and Saint Lucia 6 October 12 jurisdictions 2020 10 jurisdictions American Samoa, Anguilla, Australia, Botswana, Eswatini, Jordan, Maldives, Morocco, Namibia, Barbados, Fiji, Guam, Palau, Saint Lucia, Thailand, Turkey Panama, Samoa, Seychelles, Trinidad and Tobago, Vanuatu, US Virgin Islands 9 jurisdictions Moved from grey list to black list Anguilla American Samoa, Belize, Fiji, Guam, Oman, Samoa, Trinidad and Tobago, Vanuatu, Added to the black list Removed from the grey list US Virgin Islands Barbados Bosnia and Herzegovina, Mongolia Removed from the blacklist Cayman Islands, Oman Taxation and Customs Union 18 February 12 jurisdictions 2020 13 jurisdictions American Samoa, Cayman Anguilla, Australia, Bosnia and Herzegovina, Botswana, Eswatini, Jordan, Islands, Fiji, Guam, Palau, Maldives, Morocco, Mongolia, Namibia, Saint Lucia, Thailand and Turkey Panama, Samoa, Seychelles, Oman, Trinidad and Tobago, Vanuatu, US Virgin Islands Moved from grey list to black list Cayman Islands, Palau and Seychelles Added to the black list Removed from the grey list Panama Antigua and Barbuda, Armenia, Bahamas, Barbados, Belize, Bermuda, -

Tier 1 Countries Andorra Antigua and Barbuda Argentina Aruba Australia
Tier 1 Countries Tier 1 Countries Tier 2 Countries Tier 3 Countries Tier 4 Countries (continued) Andorra Qatar Albania Angola Afghanistan Antigua and Barbuda San Marino Algeria Anguilla Benin Argentina Saudi Arabia American Samoa Bangladesh Burkina Faso Aruba Seychelles Armenia Bhutan Burundi Australia Singapore Azerbaijan Bolivia Central African Rep. Austria Sint Maarten Belarus Cabo Verde Chad Bahamas Slovak Republic Belize Cambodia Comoros Bahrain Slovenia Bosnia/Herzegovina Cameroon Dem. Rep. of Congo Barbados South Korea Botswana Congo Eritrea Belgium Spain Brazil Cook Islands Ethiopia Bermuda St Kitts/Nevis Bulgaria Côte d’Ivoire Gambia British Virgin Islands St Martin China Djibouti Guinea Brunei Darussalam Sweden Colombia Egypt Guinea-Bissau Canada Switzerland Costa Rica El Salvador Haiti Cayman Islands Taiwan Cuba French Guiana Liberia Channel Islands Trinidad/Tobago Dominica French So. Territories Madagascar Chile Turks and Caicos Dominican Republic Georgia Malawi Croatia United Arab Emirates Ecuador Ghana Mali Curacao United Kingdom Equatorial Guinea Guadeloupe Mozambique Cyprus United States Fiji Honduras Nepal Czech Republic Uruguay Gabon India Niger Denmark Virgin Islands Grenada Indonesia North Korea Estonia Guatemala Kenya Rwanda Faroe Islands Guyana Kiribati Senegal Falkland Islands (Malvinas) Iran Kosovo Sierra Leon Finland Iraq Kyrgyz Republic Somalia France Jamaica Lao PDR South Sudan French Polynesia Jordan Lesotho Syrian Arab Republic Germany Kazakhstan Mauritania Tajikistan Gibraltar Lebanon Mayotte Tanzania Greece -

Beyond There Are Islands
BEYOND THERE ARE ISLANDS. THERE ARE EXTRAORDINARY ISLANDS. AND THEN, THERE IS ANGUILLA. ESCAPE TO THE INVITING, INTRIGUING AND TRULY INCOMPARABLE ISLAND OF ANGUILLA. Considered by many to be one of the Caribbean’s best-kept secrets, spend any amount of time here and it’ll be no secret as to why. Anguilla has become an oasis for solo travellers, couples, families and business travellers alike. Renowned for 33 powder-white beaches, turquoise waters and a culinary scene helmed by some of the Caribbean’s most talented chefs. However, the truest spirit of the island lives within its people. Proud, warm, and welcoming. VOTED PLAY THE #1 ISLAND IN THE FIND FUN IN AND CARIBBEAN OUT OF THE SUN. Spectacular sunrises and shimmering sunsets Travel + Leisure World’s Best Awards are only part of what make Anguilla so unique. Just step off the beach and into any number of island adventures to experience the other side of extraordinary. Explore the tranquil sea at night in your very own transparent kayak. Enjoy authentic Anguillian entertainment and music festivals. Roam uninhabited beaches by horseback. Or play a round of golf on a breathtaking course overlooking the sparkling Caribbean Sea. STAY You’ll find great comfort and ANGUILLA BOASTS SOME OF THE MOST EXQUISITE affordable price points at many ACCOMMODATIONS IN THE CARIBBEAN. of our island properties. Luxury and leisure can be experienced by all on Anguilla. From simple to simply spectacular, there are accommodations perfect for every traveller. Whether you choose to settle into a luxury 5-star resort, private villa, condominium residence or the small and intimate Charming Escapes Collection, you’ll feel right at home on Anguilla. -

The Arms Trade Treaty: Zimbabwe, the Democratic Republic of the Congo, and the Prospects for Arms Embargoes on Human Rights Violators
The Arms Trade Treaty: Zimbabwe, the Democratic Republic of the Congo, and the Prospects for Arms Embargoes on Human Rights Violators David B. Kopel,* Paul Gallant** and Joanne D. Eisen*** Abstract: Advocates of the proposed United Nations Arms Trade Treaty (ATT) promise that it will prevent the flow of arms to human rights violators. This Article first examines the ATT and observes that the ATT, if implemented as promised, would require dozens of additional arms embargoes, including embargoes on much of Africa. The Article then provides case studies of the current supply of arms to the dictatorship in Zimbabwe and to the warlords in the eastern Democratic Republic of the Congo (DRC). The Article argues that the ATT would do nothing to remediate the conditions that have allowed so many arms to be acquired by human rights violators. The ATT would have no more effective force than the embargoes that are already imposed by the U.N. Security Council; therefore U.N. member states, including China, which violate current Security Council embargoes, could just as well violate ATT embargoes. Accordingly, the ATT is a distraction, and human rights activists should instead examine alternative methods of addressing the problem of arms in the hands of human rights violators. At the end of this Article, there is an abstract in Spanish, and a detailed summary of the Article in French. * Adjunct Professor of Advanced Constitutional Law, Denver University Sturm College of Law. Research Director, Independence Institute, Golden, Colo.; Associate Policy Analyst, Cato Institute, Washington, D.C. We would like to thank Dave Heal (University of Michigan Law School, class of 2010) and Trevor Burrus (Denver University Sturm College of Law, class of 2010) for research assistance. -

Anguilla, Bermuda, British Virgin Islands, Cayman Isla
Antigua and Barbuda Bahamas Barbados Belize British overseas territories (Anguilla, Bermuda, British Virgin Islands, Cayman Islands, Turks and Caicos Islands, Montserrat) Canada Dominica Dominican Republic Dutch overseas territories in the Caribbean (Aruba, Curaçao, Saint Maarten, Bonaire, Saint Eustatius, Saba) French overseas departments (Martinique, Guadeloupe, Saint-Barthélemy, Saint-Martin) Grenada Guyana Haiti Jamaica St. Kitts and Nevis St. Lucia St. Vincent and the Grenadines Suriname Trinidad and Tobago Iraqi refugee who found work United States of America at the Hilton Hotel. UNHCR / T. IRWIN 264 UNHCR Global Appeal 2012-2013 North and America the Caribbean Canada and the United States of America continue to receive a expanding the authorities’ powers of detention, which could also large number of asylum-seekers. These two countries are also apply to people of concern to UNHCR. home to the largest number of resettled refugees in the world. There are fears that the global economic downturn will lead National security concerns remain the driving force behind to reduced assistance for refugee programmes both nationally policy decisions affecting people of concern to UNHCR in the and internationally. In the United States, where budget region. In Canada, legislation being proposed in response to a reduction issues are the focus of the political debate, it is not clear growing number of asylum-seekers arriving by sea, to prevent how the climate of austerity might affect refugee-assistance human smuggling and abuse of the asylum system may also programmes. High unemployment rates are also affecting people impact negatively on people who are in need of international of concern to UNHCR. -

183 Afghanistan, Albania, Algeria, Andorra, Angola, Antigua And
Session: 73rd session of General Assembly Committee: Plenary Meeting date: Wed 05 Dec 2018 Vote time: 12:27:10 PM Meeting: 45th plenary meeting Agenda item: 107 Agenda: Comprehensive Nuclear-Test-Ban Treaty Document: A/73/516 Additional document info: Draft resolution as a whole In favour: 183 Afghanistan, Albania, Algeria, Andorra, Angola, Antigua and Barbuda, Argentina, Armenia, Australia, Austria, Azerbaijan, Bahamas, Bahrain, Bangladesh, Barbados, Belarus, Belgium, Belize, Benin, Bhutan, Bolivia (Plurinational State of), Bosnia and Herzegovina, Botswana, Brazil, Brunei Darussalam, Bulgaria, Burkina Faso, Burundi, Cabo Verde, Cambodia, Cameroon, Canada, Central African Republic, Chile, China, Colombia, Comoros, Congo, Costa Rica, Côte d'Ivoire, Croatia, Cuba, Cyprus, Czech Republic, Democratic Republic of the Congo, Denmark, Djibouti, Dominica, Dominican Republic, Ecuador, Egypt, El Salvador, Equatorial Guinea, Eritrea, Estonia, Eswatini, Ethiopia, Fiji, Finland, France, Gabon, Gambia, Georgia, Germany, Ghana, Greece, Grenada, Guatemala, Guinea, Guinea-Bissau, Guyana, Haiti, Honduras, Hungary, Iceland, Indonesia, Iran (Islamic Republic of), Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kazakhstan, Kenya, Kuwait, Kyrgyzstan, Lao People's Democratic Republic, Latvia, Lebanon, Lesotho, Liberia, Libya, Liechtenstein, Lithuania, Luxembourg, Madagascar, Malawi, Malaysia, Maldives, Mali, Malta, Marshall Islands, Mauritania, Mexico, Micronesia (Federated States of), Monaco, Mongolia, Montenegro, Morocco, Mozambique, Myanmar, Namibia,