Zygoptera: Coenagrionidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Foraging Behavior of Anax Junius (Odonata: Aeschnidae) and Its Potential As a Behavioral Endpoint in Pesticide Testing Sandra Kaye Brewer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1997 The foraging behavior of Anax junius (Odonata: Aeschnidae) and its potential as a behavioral endpoint in pesticide testing Sandra Kaye Brewer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Environmental Sciences Commons, Fresh Water Studies Commons, Toxicology Commons, and the Zoology Commons Recommended Citation Brewer, Sandra Kaye, "The foraging behavior of Anax junius (Odonata: Aeschnidae) and its potential as a behavioral endpoint in pesticide testing " (1997). Retrospective Theses and Dissertations. 11445. https://lib.dr.iastate.edu/rtd/11445 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. UMI MICROFILMED 1997 INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter fiice, while others may be from any type of computer printer. The quality of this reprcduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

The Superfamily Calopterygoidea in South China: Taxonomy and Distribution. Progress Report for 2009 Surveys Zhang Haomiao* *PH D
International Dragonfly Fund - Report 26 (2010): 1-36 1 The Superfamily Calopterygoidea in South China: taxonomy and distribution. Progress Report for 2009 surveys Zhang Haomiao* *PH D student at the Department of Entomology, College of Natural Resources and Environment, South China Agricultural University, Guangzhou 510642, China. Email: [email protected] Introduction Three families in the superfamily Calopterygoidea occur in China, viz. the Calo- pterygidae, Chlorocyphidae and Euphaeidae. They include numerous species that are distributed widely across South China, mainly in streams and upland running waters at moderate altitudes. To date, our knowledge of Chinese spe- cies has remained inadequate: the taxonomy of some genera is unresolved and no attempt has been made to map the distribution of the various species and genera. This project is therefore aimed at providing taxonomic (including on larval morphology), biological, and distributional information on the super- family in South China. In 2009, two series of surveys were conducted to Southwest China-Guizhou and Yunnan Provinces. The two provinces are characterized by karst limestone arranged in steep hills and intermontane basins. The climate is warm and the weather is frequently cloudy and rainy all year. This area is usually regarded as one of biodiversity “hotspot” in China (Xu & Wilkes, 2004). Many interesting species are recorded, the checklist and photos of these sur- veys are reported here. And the progress of the research on the superfamily Calopterygoidea is appended. Methods Odonata were recorded by the specimens collected and identified from pho- tographs. The working team includes only four people, the surveys to South- west China were completed by the author and the photographer, Mr. -

The Impacts of Urbanisation on the Ecology and Evolution of Dragonflies and Damselflies (Insecta: Odonata)
The impacts of urbanisation on the ecology and evolution of dragonflies and damselflies (Insecta: Odonata) Giovanna de Jesús Villalobos Jiménez Submitted in accordance with the requirements for the degree of Doctor of Philosophy (Ph.D.) The University of Leeds School of Biology September 2017 The candidate confirms that the work submitted is her own, except where work which has formed part of jointly-authored publications has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. The work in Chapter 1 of the thesis has appeared in publication as follows: Villalobos-Jiménez, G., Dunn, A.M. & Hassall, C., 2016. Dragonflies and damselflies (Odonata) in urban ecosystems: a review. Eur J Entomol, 113(1): 217–232. I was responsible for the collection and analysis of the data with advice from co- authors, and was solely responsible for the literature review, interpretation of the results, and for writing the manuscript. All co-authors provided comments on draft manuscripts. The work in Chapter 2 of the thesis has appeared in publication as follows: Villalobos-Jiménez, G. & Hassall, C., 2017. Effects of the urban heat island on the phenology of Odonata in London, UK. International Journal of Biometeorology, 61(7): 1337–1346. I was responsible for the data analysis, interpretation of results, and for writing and structuring the manuscript. Data was provided by the British Dragonfly Society (BDS). The co-author provided advice on the data analysis, and also provided comments on draft manuscripts. -

Dragonf Lies and Damself Lies of Europe
Dragonf lies and Damself lies of Europe A scientific approach to the identification of European Odonata without capture A simple yet detailed guide suitable both for beginners and more expert readers who wish to improve their knowledge of the order Odonata. This book contains images and photographs of all the European species having a stable population, with chapters about their anatomy, biology, behaviour, distribution range and period of flight, plus basic information about the vagrants with only a few sightings reported. On the whole, 143 reported species and over lies of Europe lies and Damself Dragonf 600 photographs are included. Published by WBA Project Srl CARLO GALLIANI, ROBERTO SCHERINI, ALIDA PIGLIA © 2017 Verona - Italy WBA Books ISSN 1973-7815 ISBN 97888903323-6-4 Supporting Institutions CONTENTS Preface 5 © WBA Project - Verona (Italy) Odonates: an introduction to the order 6 WBA HANDBOOKS 7 Dragonflies and Damselflies of Europe Systematics 7 ISSN 1973-7815 Anatomy of Odonates 9 ISBN 97888903323-6-4 Biology 14 Editorial Board: Ludivina Barrientos-Lozano, Ciudad Victoria (Mexico), Achille Casale, Sassari Mating and oviposition 23 (Italy), Mauro Daccordi, Verona (Italy), Pier Mauro Giachino, Torino (Italy), Laura Guidolin, Oviposition 34 Padova (Italy), Roy Kleukers, Leiden (Holland), Bruno Massa, Palermo (Italy), Giovanni Onore, Quito (Ecuador), Giuseppe Bartolomeo Osella, l’Aquila (Italy), Stewart B. Peck, Ottawa (Cana- Predators and preys 41 da), Fidel Alejandro Roig, Mendoza (Argentina), Jose Maria Salgado Costas, Leon (Spain), Fabio Pathogens and parasites 45 Stoch, Roma (Italy), Mauro Tretiach, Trieste (Italy), Dante Vailati, Brescia (Italy). Dichromism, androchromy and secondary homochromy 47 Editor-in-chief: Pier Mauro Giachino Particular situations in the daily life of a dragonfly 48 Managing Editor: Gianfranco Caoduro Warming up the wings 50 Translation: Alida Piglia Text revision: Michael L. -

A Decrease in Endemic Odonates in the Ogasawara Islands, Japan
「森林総合研究所研究報告」(Bulletin of FFPRI), Vol.4, No.1 (No.394), 45 - 51, Mar. 2005 論 文(Original article) A decrease in endemic odonates in the Ogasawara Islands, Japan YOSHIMURA Mayumi 1)* and OKOCHI Isamu 2) Abstract There are many endemic species in the Japanese Ogasawara Islands. However, many of these endemic species are likely to disappear as a result of reduction of habitat and the introduction of exotic species. Odonates are included within this category of species at risk. If the decrease in endemic odonates is due to a decrease in aquatic habitat, we have only to provide arti” fi cial ponds to conserve these species. In this study, we provided artifi cial ponds as a habitat for odonates in Chichi-jima and Ani-jima, Ogasawara Islands. We then examined the possibility of protection and enhancement of odonate populations. Endemic odonates were found in the natural ponds of Ani-jima and Ototo-jima. In Ani-jima, they could be collected both in the artifi cial and natural ponds. The artifi cial pond could provide habitat for endemic odonates. However, in Chichi-jima, few odonates could be collected both in the artifi cial and natural ponds. Here, invasive species, such as Gambusia affi nis and Anolis carolinensis, are found, which considered to prey upon odonate larvae and adults. Extermination of invasive species may be necessary to conserve the endemic odonates in Chichi-jima. Key words : Anolis carolinensis, endemic species, oceanic islands, odonates, Ogasawara Islands, predator, conservation Introduction in Chichi-jima. In Japan, the Ogasawara Islands consist of many This decline has been blamed on environmental small islands, including Chichi-jima, Ani-jima, Ototo- destruction. -

Northland Lakes Water Quality and Ecology State and Trends 2007-2011
Northland Lakes water quality and ecology State and trends 2007-2011 2 Northland Lakes water quality and ecology State and trends 2007-2011 John Ballinger Emma Simpson Carol Nicholson Jean-Charles Perquin September 2013 3 Executive Summary The Northland region has a large number of lakes, a large proportion of which were formed by dune activity and are classed as dune lakes. Many Northland Lakes are of national importance representing some of the few remaining lakes with intact native flora and containing a number of rare and endangered plant and animal species. This report is a companion report to the five yearly State of the Environment Report 2012, detailing the state and trends of water quality for 28 lakes in the current Northland lake monitoring network from 2007 to 2011. The report also provides a summary of relevant ecological data from the annual report produced by the National Institute of Water and Atmospherics (NIWA) which updates the ecological status of 86 lakes in the Northland region (Wells & Champion, 2011). Both the water quality and ecological data was used to develop the Northland Lake Monitoring Strategy (Champion, de Winter 2012) which aims to prevent the deterioration of water quality and biodiversity values in Northland’s lakes and help to restore these valuable ecosystems. Water quality state is measured using the nationally recognised indicators Lake Trophic Level Index (TLI) and Lake Submerged Plant Indicator (LakeSPI). Trend analysis was carried out on all variables with more than three years’ worth of data. A Biodiversity Assessment gives each lake an overall ranking taking into account many indicators including water quality, endangered species identified, wetland extent, species composition, submerged vegetation abundance and composition (including the Lake SPI), water birds, fish and aquatic invertebrate presence and abundance (Wells and Chapman, 2011). -

Download Download
The Distribution and Relative Seasonal Abundance of the Indiana Species of Agrionidae (Odonata: Zygoptera) B. Elwood Montgomery, Purdue University In a previous paper (Montgomery, 1942) the relative seasonal abundance of the adults of the 16 species of Enallagma known from Indiana, based upon the frequency of collection during 41 years (1900- 1940 inclusive) was indicated. That study is extended here to include the remaining 16 species of the family Agrionidae recorded from the state. <I3jXEEEKE> Fig. 1. The range of the flight season (or period of adult life) and the relative seasonal abundance of 16 species of Agrionidae (genera Argia, Nehalen- nia, Amphiagrion, Chromagrion, Ischnura and Anonialagrion ) in Indiana. Col- lections made from 1900 to 1940 inclusive were tabulated by thirds of months and the graphs constructed from the resulting frequency distributions. Numbers near each bar indicate the number of collections of each species in each third of a month ; where no number is given the number of collections is one. 179 180 Indiana Academy of Science Records of almost all Odonata collected or observed in Indiana since 1900 have been preserved in the note books of the late E. B. Williamson and of the author. The records for the species of the genera Argia, Nehalennia, Amphiagrion, Chromagrion, Ischnura, and Anomalagrion have been tabulated and the accompanying chart (Figure 1) indicates the relative abundance of the different species throughout the season of adult flight. As this study applies only to adults, all references to seasonal range and abundance in this paper refer to the period of adult flight. The records of captures (or observations) were tabulated by thirds of months and the time-frequency graph for each species was con- structed by plotting the frequency for each third at the midpoint (5th, 15th and 25th of the month, respectively) of the third of the time axis. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Argia the News Journal of the Dragonfly Society of the Americas
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 22 17 December 2010 Number 4 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 22, No. 4, 17 December 2010 In This Issue .................................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 Minutes of the 2010 Annual Meeting of the Dragonfly Society of the Americas, by Steve Valley ............................2 2010 Treasurer’s Report, by Jerrell J. Daigle ................................................................................................................2 Enallagma novaehispaniae Calvert (Neotropical Bluet), Another New Species for Arizona, by Rich Bailowitz ......3 Photos Needed ............................................................................................................................................................3 Lestes australis (Southern Spreadwing), New for Arizona, by Rich Bailowitz ...........................................................4 Ischnura barberi (Desert Forktail) Found in Oregon, by Jim Johnson ........................................................................4 Recent Discoveries in Montana, by Nathan S. Kohler ...............................................................................................5 -
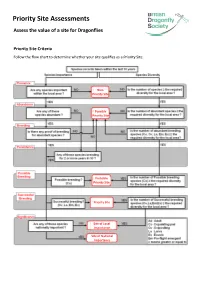
Priority Site Assessments Assess the Value of a Site for Dragonflies
Priority Site Assessments Assess the value of a site for Dragonflies Priority Site Criteria Follow the flow chart to determine whether your site qualifies as a Priority Site. Non- Priority Site Possible Priority Site Probable Priority Site Priority Site Site of Local Importance Site of National Importance Nationally Important Species List Azure Hawker, Aeshna caerulea Scarce Chaser, Libellula fulva Brilliant Emerald, Somatochlora metallica Scarce Emerald Damselfly, Lestes dryas Common Clubtail, Gomphus vulgatissimus Southern Damselfly, Coenagrion mercuriale Northern Emerald, Somatochlora arctica Small Red Damselfly, Ceriagrion tenellum Norfolk Hawker, Aeshna isosceles Variable Damselfly, Coenagrion pulchellum Northern Damselfly, Coenagrion hastulatum White-faced Darter, Leucorrhinia dubia Scarce Blue-tailed Damselfly, Ischnura pumilio Locally Important Species List and Diversity Criteria The diversity criteria is the minimum number of species that need to have been recorded within the site, within the last 10 years, for it to qualify. Counties Locally Important Species Diversity criteria Cornwall White-legged Damselfly, Platycnemis pennipes 11 Isles of Scilly Ruddy Darter, Sympetrum sanguineum Devon White-legged Damselfly, Platycnemis pennipes 14 Red-eyed Damselfly, Erythromma najas Hairy Dragonfly, Brachytron pratense Downy Emerald, Cordulia aenea Ruddy Darter, Sympetrum sanguineum Dorset White-legged Damselfly, Platycnemis pennipes 14 Red-eyed Damselfly, Erythromma najas Downy Emerald, Cordulia aenea Somerset Common Hawker, Aeshna juncea -

Richness and Diversity of Odonates of the Agricultural College and Research Institute, Vazhavachanur, Tamilnadu, India
#0# Acta Biologica 27/2020 | www.wnus.edu.pl/ab | DOI: 10.18276/ab.2020.27-06 | strony 57–65 Richness and diversity of odonates of the agricultural college and research institute, Vazhavachanur, Tamilnadu, India Vaithiyanathan Radhakrishnan,1 Ramanathan Arulprakash,2 Iyappan Parivarthani,3 Selvarasu Ponnivalavan,3 Mohan Priyadharshini,3 Muthaiyan Pandiyan4 1 Agricultural College and Research Institute, Vazhavachanur – 606 753, Thiruvannamalai District, Tamil Nadu, India 2 Seeds Centre, Tamil Nadu Agricultural University, Coimbatore – 641 003, Tamil Nadu, India 3 Agricultural College and Research Institute, Vazhavachanur – 606 753, Thiruvannamalai District, Tamil Nadu, India 4 Agricultural College and Research Institute, Vazhavachanur – 606 753, Thiruvannamalai District, Tamil Nadu, India Corresponding Authora e-mail: [email protected], [email protected] Keywords Vazhavachanur, Dragonfly, Damselfy, Libellulidae and Coenagrionidae Abstract Investigations on the diversity of Odonata in and around the Agricultural College and Research Institute, Vazhavachanur, Tamil Nadu, India were studied. Eight locations were selected, of which sixteen Odonata species were recorded. In total, eleven dragonfly and five damselfly species were identified from Thiruvannamalai district, Tamil Nadu, India.Pantala flavescens, Diplacodes trivialis, Brachythemis contaminata and Ischnura aurora were recorded from all eight locations. Trithemis pallidinervis and Agriocnemis pygmaea were recorded from seven locations except from the farm pond and the open stretch area. Rhyothemis variegata was recorded only at the open stretch area. The results clearly show that, Odonates have specific habitat preferences for their growth and development. Four families Libellulidae, Gomphidae, Aeshnidae and Coenagrionidae were observed and collected during the study. Libellulidae were the most abundant family (56.25%) and comprised of 9 species, followed by Coenagrionidae (31.25%) with 5 species. -

Conservation Advice for the Karst Springs and Associated Alkaline Fens of the Naracoorte Coastal Plain Bioregion
The Threatened Species Scientific Committee provided their advice to the Minister on 31 July 2020. The Minister approved this Conservation Advice on 3 December 2020 and agreed that no recovery plan is required at this time. Conservation Advice1 for the Karst springs and associated alkaline fens of the Naracoorte Coastal Plain Bioregion This document combines the approved conservation advice and listing assessment for the threatened ecological community. It provides a foundation for conservation action and further planning. Karst springs and alkaline fens, Ewen Ponds © Copyright, Anthony Hoffman Conservation Status The Karst springs and associated alkaline fens of the Naracoorte Coastal Bioregion is listed in the Endangered category of the threatened ecological communities list under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act). The ecological community was assessed by the Threatened Species Scientific Committee, who found it to be eligible for listing as Endangered and recommended that a recovery plan is not required at this time. The Committee’s assessment and recommendations are at Section 6. The Committee’s assessment of the eligibility against each of the listing criteria is: Criterion 1: Vulnerable Criterion 2: Endangered Criterion 3: Insufficient data Criterion 4: Endangered Criterion 5: Insufficient data Criterion 6: Insufficient data The main factors that make the threatened ecological community eligible for listing in the Endangered category are its historic losses to drainage, clearing and resulting fragmentation, and ongoing threats to its integrity and function, particularly from hydrological changes. The Karst springs and associated alkaline fens of the Naracoorte Coastal Plain Bioregion occurs within country (the traditional lands) of the Boandik and the Gunditjmara peoples.