A Meta-Analysis and Review of Sex Differences in Disease and Immunity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Torrey Peters Has Written the Trans Novel Your Book Club Needs to Read Now P.14
Featuring 329 Industry-First Reviews of Fiction, Nonfiction, Children'sand YA books KIRKUSVOL. LXXXIX, NO. 1 | 1 JANUARY 2021 REVIEWS Torrey Peters has written the trans novel your book club needs to read now p.14 Also in the issue: Lindsay & Lexie Kite, Jeff Mack, Ilyasah Shabazz & Tiffany D. Jackson from the editor’s desk: New Year’s Reading Resolutions Chairman BY TOM BEER HERBERT SIMON President & Publisher MARC WINKELMAN John Paraskevas As a new year begins, many people commit to strict diets or exercise regimes # Chief Executive Officer or vow to save more money. Book nerd that I am, I like to formulate a series MEG LABORDE KUEHN of “reading resolutions”—goals to help me refocus and improve my reading [email protected] Editor-in-Chief experience in the months to come. TOM BEER Sometimes I don’t accomplish all that I hoped—I really ought to have [email protected] Vice President of Marketing read more literature in translation last year, though I’m glad to have encoun- SARAH KALINA [email protected] tered Elena Ferrante’s The Lying Life of Adults (translated by Ann Goldstein) Managing/Nonfiction Editor and Juan Pablo Villalobos’ I Don’t Expect Anyone To Believe Me (translated by ERIC LIEBETRAU Daniel Hahn)—but that isn’t exactly the point. [email protected] Fiction Editor Sometimes, too, new resolutions form over the course of the year. Like LAURIE MUCHNICK many Americans, I sought out more work by Black writers in 2020; as a result, [email protected] Tom Beer Young Readers’ Editor books by Claudia Rankine, Les and Tamara Payne, Raven Leilani, Deesha VICKY SMITH [email protected] Philyaw, and Randall Kenan were among my favorites of the year. -

The Record 2019/20
The Record 2019/20 The Record 2019/20 contents 5 Letter from the Warden 6 Fellows and Academic Staff 9 Fellowship Elections and Appointments 10 Non-academic Staff 13 JCR and MCR Committees 14 Matriculation 20 Undergraduate Scholarships 22 College Awards and Prizes 24 Academic Distinctions 27 Higher Degrees 28 Fellows’ Publications 36 Sports and Games 40 Clubs and Societies 41 The Chapel 41 Parishes Update 41 Bursar’s Update 42 Gifts to the Library and Archive 43 Fellows’ Obituaries 46 Alumni Obituaries 62 News of Alumni letter from the warden When I wrote this letter last year we had just had the official opening of the H B Allen Centre by HRH the Duke of Cambridge at the beginning of what we expected to be a marvellous year of celebration of the College’s 150th anniversary. The impact of COVID-19 means that this year’s perspective is gloomier. Over the summer we initiated a redundancy programme for our non-academic staff in response to the financial impact and the operational consequences of the pandemic. We also spent a great deal of time planning for the return of students for Michaelmas Term with as much attention to sustaining the positive aspects of their experience as public health restrictions will allow. Unsurprisingly there is an atmosphere of uncertainty about how the external context will influence what happens. We recognise that we are unlikely to see a full return to anything like our previous normality in the course of this academic year. However, I do need to record changes in the Fellowship in the usual way. -

Male Sex Identified by Global COVID-19 Meta-Analysis As a Risk
ARTICLE https://doi.org/10.1038/s41467-020-19741-6 OPEN Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission Hannah Peckham 1,2, Nina M. de Gruijter 1,2, Charles Raine 2, Anna Radziszewska 1,2, ✉ Coziana Ciurtin 1,2, Lucy R. Wedderburn 1,3,4, Elizabeth C. Rosser 1,2,7, Kate Webb 5,6,7 & ✉ Claire T. Deakin 1,3,4,7 1234567890():,; Anecdotal evidence suggests that Coronavirus disease 2019 (COVID-19), caused by the coronavirus SARS-CoV-2, exhibits differences in morbidity and mortality between sexes. Here, we present a meta-analysis of 3,111,714 reported global cases to demonstrate that, whilst there is no difference in the proportion of males and females with confirmed COVID- 19, male patients have almost three times the odds of requiring intensive treatment unit (ITU) admission (OR = 2.84; 95% CI = 2.06, 3.92) and higher odds of death (OR = 1.39; 95% CI = 1.31, 1.47) compared to females. With few exceptions, the sex bias observed in COVID-19 is a worldwide phenomenon. An appreciation of how sex is influencing COVID-19 outcomes will have important implications for clinical management and mitigation strategies for this disease. 1 Centre for Adolescent Rheumatology Versus Arthritis at UCL, UCLH, GOSH, London, UK. 2 Centre for Rheumatology Research, Division of Medicine, UCL, London, UK. 3 Infection, Immunity and Inflammation Research and Teaching Department, UCL Great Ormond Street Institute of Child Health, London, UK. 4 NIHR Biomedical Research Centre at Great Ormond Street Hospital, London, UK. -

Foreign Correspondents in Vietnam Laura Palmer & Jurate Kazickas
Foreign Correspondents in Vietnam Laura Palmer & Jurate Kazickas Some were sent as foreign correspondents by numbers were small. The first arrived in 1961, news organizations back in the States, others the last left in 1975. Eighty is a conservative arrived on a one-way ticket purchased with estimate. The actual number could be twice winnings from being a game-show contestant. that when stringers, freelancers and photo Some had impressive journalism credentials journalists are included. We won every major and long track records, others got their first award, including the Pulitzer Prize. press pass in Vietnam. The one thing every woman had who covered the war in Vietnam We did not agree politically. There were was the determination to do her damndest. women who supported the war and women who violently opposed it. We were not unan- Risks, we took. Fear, we hid. Courage we imous in anything except perhaps our mustered up. Fatigue we ignored. Strength we passionate pride for the jobs we did. Sure, found, often more than we ever thought we there were legends like Frances Fitzgerald, had. Laughter helped heal and what wasn’t Gloria Emerson, Marguerite Higgins, written down in our notebooks or recorded on Georgie Ann Geyer, Kate Webb, Elizabeth tape is still there, in our heart-of-hearts. Pond, Beverly Deepe, Liz Trotta and Ann | Bryan Mariano. Ability, determination and humor became a strategy for making them accept us. Them were And Dickey Chapelle, who was killed on the generals who felt women belonged some- November 4, 1965 while out on patrol with the where other than wherever the war was. -

Oped I Don't Want My Role Models Erased
3/20/2021 Opinion | I Don’t Want My Role Models Erased - The New York Times I Don’t Want My Role Models Erased This is how women who covered Vietnam were marginalized in the warʼs history. By Elizabeth Becker Ms. Becker, a former Times correspondent, reported from Cambodia in the mid- 1970s. She is the author of “You Do Not Belong Here,” a biography of the women war correspondents of the Vietnam War. March 20, 2021 Vietnam, the 10-year American fiasco that foreshadowed the disastrous forever wars of today, was written into history as it happened. The war’s most famous chroniclers — David Halberstam, Neil Sheehan and Malcolm Browne — were young men, all. But women journalists were there, too, reporting the war and risking their lives to bring back the story. I know because I was there learning from them. I arrived late in January 1973 and profited from the opening they made, and the example they set, by focusing on the humane questions of war. Kate Webb, a revered combat reporter, taught me how to measure a bomb crater with my feet when we covered the carpet bombing of Cambodia. Ms. Webb rose to become bureau chief for United Press International in the war zone, covering more battles than most of her male colleagues. She was captured by the North Vietnamese in Cambodia in 1971 and held for 23 days. When she came out alive, her story was front-page news. An Agence France-Press prize for journalists working in “perilous or difficult conditions” in Asia was named in her honor. -

Female War Correspondents in Vietnam: a Turning Point for Women in American Journalism
FEMALE WAR CORRESPONDENTS IN VIETNAM: A TURNING POINT FOR WOMEN IN AMERICAN JOURNALISM By Natalia J. Haller A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment Of the Requirements for the Degree Master of Arts in Social Science Emphasis: Teaching American History May, 2006 FEMALE CORRESPONDENTS IN THE VIETNAM WAR: A TURNING POINT FOR WOMEN IN AMERICAN JOURNALISM by Natalia J. Haller Approved by the Master’s Thesis Committee: Delores McBroome, Major Professor Date Gayle Olson-Raymer, Committee Member Date Rodney Sievers, Committee Member Date Delores McBroome, Graduate Coordinator Date Donna E. Schafer, Dean for Research and Graduate Studies Date ABSTRACT Considering the amount of literature written on the Vietnam War, it is confounding that female war correspondents have failed to make a significant entry into historical accounts of the conflict. Part of the challenge when searching for literature on the female war correspondent in Vietnam is that historically, war and journalism have been considered a man’s area of expertise. Much of the literature written about reporters in Vietnam reflects this sentiment. This perception was transformed during the Vietnam War by an unprecedented number of courageous women who broke the stereotypes to become successful wartime correspondents. Unrestricted access to the fighting proved to be an opportunity for women journalists. Four hundred and sixty seven women became accredited during the war, of which 267 were American. The purpose of my research was to review the literature on various factors that created opportunity for women journalists in Vietnam and develop a prosopography of the female war correspondent. -

Vietnam Ebook Free Download
VIETNAM PDF, EPUB, EBOOK Lonely Planet,Iain Stewart,Brett Atkinson,Anna Kaminski,Jessica Lee,Nick Ray,Benedict Walker | 520 pages | 16 Aug 2016 | Lonely Planet Publications Ltd | 9781743218723 | English | Hawthorn, Victoria, Australia Vietnam PDF Book Europe Since An Encyclopedia. Archived from the original PDF on 17 October The Tongking Gulf Through History. Bruce Between July and December , more than , U. Footprint Travel Guides. John Benjamins Publishing Company. The range based on the figures above extends from a minimum of 1. Along the coast of central Vietnam are regosols soft, undeveloped soils and noncalcic brown soils. Communist forces were under a single command structure set up in Much like the general historiography of the war, discussion of myth has focused on U. New York: Webster's New World. In the reserve was approximately 4. Journal of Environmental and Public Health. Article Contents. Routledge Curzon. Please help improve this article by adding citations to reliable sources. Ground forces also had access to B and F-4 Phantom II and other aircraft to launch napalm , white phosphorus , tear gas , chemical weapons , precision-guided munition and cluster bombs. Retrieved 2 May Spector Ronald H. Luong and Khai had led Vietnam since and were instrumental in Vietnam's two-decades-long transition to a market economy, called doi moi, or renovation. Most are farmers. This paved the way for successive imperial dynasties as the nation expanded geographically southward until the Indochina Peninsula was colonised by the French in the late 19th century. University of Michigan. Southeast Asia in the New International Era. The later years of the war saw increased physical and psychological deterioration among American soldiers—both volunteers and draftees—including drug use , post-traumatic stress disorder PTSD , mutinies and attacks by soldiers against officers and noncommissioned officers. -
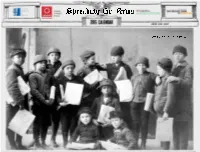
2015 SPREADING the NEWS.Pdf
Spreading the News Newsboys, Ann Arbor, Michigan,1892. I am very pleased to introduce the CUNY/New York Times in College 2015 calendar, Spreading the News, a history of journalism in the United States. Published in a time of rapid changes in the industry, it is a timely and welcome contribution to the history of our nation’s fourth estate. The Founding Fathers saw the danger of government censorship during the War for Independence and believed it important to enshrine freedom of the press into the First Amendment. The calendar explores the origins of press freedom and how it is periodically under attack, especially during times of crisis and war. The calendar will also explore how changes in society and technol- ogy have transformed how publishers, editors and journalists produce the news including the rise of the penny press, the development of radio and television, and the rise of the Internet. Media have also been powerful sources of social change. Loyalists and Patriots used colonial newspa- pers to spread their views. In 1831, William Lloyd Garrison published the Liberator to support abolitionism. In the early 20th century, muckrakers turned their focus on government corruption and the danger of corporate monopolies. More recently, Rachel Carson made Americans aware of the environmental dangers of DDT in her book Silent Spring. In the 21st cen- tury, the Internet and social media have become both sources of news and the means to build social movements. Spreading the News is the 12th calendar/website developed in a part- nership between the City University of New York and The New York Times in Education with the support of JPMorgan Chase, Produced by the LaGuardia and Wagner Archives at LaGuardia Community College, it is emblematic of CUNY’s educational mission and commitment to public service. -

China Sees Drop in New Virus Cases, but More Deaths Abroad
World Friday, February 21, 2020 05 Trump ignores outcry China sees drop in new virus and tweets on ally’s cases, but more deaths abroad court sentencing AFP Fatalities in China hit 2,118 but toll abroad climbs to 11 after confirmed deaths in Japan and S Korea WASHINGTON PRESIDENT Donald Trump AFP on Thursday brazenly ignored BEIJING his attorney general’s plea to stop tweeting about ongoing CHINA on Thursday touted court cases, again express- a big drop in new virus infec- ing displeasure with how his tions as proof its epidemic con- longtime ally Roger Stone is trol efforts are working, but the being treated in court. toll grew abroad with deaths in The president questioned Japan and South Korea. the “fairness” in a tweet to his Roger Stone Fatalities in China hit 2,118 nearly 73 million followers, as 114 more people died, but right as a Washington, DC, ration between the White health officials reported the federal judge was opening House and the judiciary -- al- lowest number of new cases in Stone’s sentencing hearing. legedly with the aim of pro- nearly a month, including in Roger Stone was jailed tecting himself and his allies. hardest-hit Hubei province. for 40 months on Thursday Stone, a veteran Repub- More than 74,000 peo- for impeding a congression- lican operative and one of ple have been infected by the al investigation, in a case Trump’s oldest confidants, new coronavirus in China, that ignited a firestorm over was convicted in November and hundreds more in over the US president’s political of lying to Congress, tam- 25 countries. -

Anthony Veasna So Remembered Holmes Chan Hong Kong Spirit Richard Heydarian Asian Revolutionaries Anthony Tao Hutong Secrets
Anthony Veasna So remembered ASIAN LITERATURE FEBRUARY–APRIL 2021 Holmes Chan Anthony Tao Hong Kong spirit Hutong secrets Richard Heydarian Tse Wei Lim Asian revolutionaries Hawker culture 32 9 772016 012803 VOLUME 6, NUMBER 2 FEBRUARY–APRIL 2021 HISTORY 3 Thomas A. Bass Kissinger and Ellsberg in Vietnam TAIWAN 7 Michael Reilly Between two whales POETRY 8 Maw Shein Win ‘Phone booth’, ‘Shops’, ‘Restaurant’, ‘Factory’, ‘Eggs’, ‘Huts’ INTERVIEW 9 Andrew Quilty Mullah Abdul Rahman, Taliban commander NOTEBOOK 12 Yuen Chan In China’s grip ASIA 13 Richard Heydarian Underground Asia: Global Revolutionaries and the Assault on Empire by Tim Harper MYANMAR 14 David Scott Mathieson The Burmese Labyrinth: A History of the Rohingya Tragedy by Carlos Sardina Galache HONG KONG 15 Holmes Chan Defiance: Photographic Documentary of Hong Kong’s Awakening; Voices JOURNALISM 16 Martin Stuart-Fox You Don’t Belong Here: How Three Women Rewrote the Story of War by Elizabeth Becker CHINA 18 Anne Stevenson-Yang Anxious China: Inner Revolution and Politics of Psychotherapy by Li Zhang AUSTRALIA 19 Jeff Sparrow The Carbon Club: How a Network of Influential Climate Sceptics, Politicians and Business Leaders Fought to Control Australia’s Climate Policy by Marian Wilkinson MALAYSIA 20 Charles Brophy Automation and the Future of Work by Aaron Benanav; Work in an Evolving Malaysia: The State of Households 2020, Part II by Khazanah Research Institute RELIGION 21 Christopher G. Moore Finding the Heart Sutra; Guided by a Magician, an Art Collector and Buddhist Sages from -

Three Held with Dozens of Unlicensed Firearms
SUBSCRIPTION SATURDAY, JULY 5, 2014 RAMADAN 7, 1435 AH No: 16217 Kurds seeking Djokovic downs independence Dimitrov to reach Emsak: 03:09 Fajer: 03:19 referendum Wimbledon final Dohr: 11:52 Asr: 15:26 Maghreb: 18:51 11 44 Eshaa: 20:22 Three held with dozens of unlicensed firearms Max 48º 150 Fils Min 29º 26 pistols, 19 machine guns, 5 hunting rifles seized By Hanan Al-Saadoun KUWAIT: Three arms dealers were arrested yesterday with possession of a large number of unlicensed firearms in addition to drugs. The weapons found with the suspects include 1 bazooka, 26 pistols, 19 machine guns, 5 hunting rifles, and 1 shotgun, in addition to live ammunition. Police also found 150 grams of shabu (methamphetamine) and 150 drug pills in the suspects’ possession. The arrest happened following investigations about a Kuwaiti man with possession of an assortment of weapons. Drug Control General Department detectives moved to the man’s house in Riqqa with a warrant based on information that he was in possession of drugs. They raided the house and found 2 machine guns, 3 pistols, 4 rifles and 1 shotgun, in addition to the drugs. The man gave information of a Gulf national that he said provided him with the contrabands. Police raided the sec- ond suspect’s house and found 15 pistols, 6 machine guns and a large number of live ammunition. Police then raided the house of a third suspect, a Kuwaiti man, after the second suspect gave his identity. A bazooka, 11 machine guns, 1 rifle, 8 pistols and a large number of live ammunition were found inside. -

Feb. 25 Bulletin
Feb. 25 Bulletin Bulletin Feb. 25, 2021 Greetings! We hope you enjoy this issue of the Bulletin, which features three upcoming events in March and April, a recap and video from our Feb. 12 Book Night with Elizabeth Becker, the third and final installment of Irwin Chapman’s remembrance article for the OPC, plus resources and updates on club members. Upcoming OPC Events March 18: Showdown Taiwan: The United States and China Face a Crucial Confrontation Over the Democratically Governed Island Time: 7:00 p.m. Eastern Time, (7:00 a.m. Taiwan time on March 19) The government of Xi Jinping is increasing military and other pressures on Taiwan in an apparent attempt to https://myemail.constantcontact.com/Feb--25-Bulletin.html?soid=1102853718750&aid=qwiIwOXvBXU[2/27/2021 3:28:21 PM] Feb. 25 Bulletin force it to submit to the mainland’s control. Chinese military aircraft including bombers are regularly testing Taiwan's air defenses and Beijing's global pressure campaign has kept Taiwan out of the World Health Organization and other important international forums. The new administration of President Joseph Biden has signaled strong support for Taiwan, but it remains to be seen how it will conduct military, diplomatic and economic exchanges with Taiwan that risk provoking strong responses from Beijing. Will it maintain a policy of "strategic ambiguity" regarding its commitment to Taiwan's defense? Does it still possess the military power to successfully defend Taiwan in view of China’s strengthened military and technology sectors? The panel will feature: Si-Fu Ou, Director, Division of Chinese Politics, Military and Warfighting Concepts, Taiwan’s Institute for National Defense and Security Research.