Supplemental Figure 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbial Community Structure Dynamics in Ohio River Sediments During Reductive Dechlorination of Pcbs
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2008 MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS Andres Enrique Nunez University of Kentucky Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Nunez, Andres Enrique, "MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS" (2008). University of Kentucky Doctoral Dissertations. 679. https://uknowledge.uky.edu/gradschool_diss/679 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Andres Enrique Nunez The Graduate School University of Kentucky 2008 MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Agriculture at the University of Kentucky By Andres Enrique Nunez Director: Dr. Elisa M. D’Angelo Lexington, KY 2008 Copyright © Andres Enrique Nunez 2008 ABSTRACT OF DISSERTATION MICROBIAL COMMUNITY STRUCTURE DYNAMICS IN OHIO RIVER SEDIMENTS DURING REDUCTIVE DECHLORINATION OF PCBS The entire stretch of the Ohio River is under fish consumption advisories due to contamination with polychlorinated biphenyls (PCBs). In this study, natural attenuation and biostimulation of PCBs and microbial communities responsible for PCB transformations were investigated in Ohio River sediments. Natural attenuation of PCBs was negligible in sediments, which was likely attributed to low temperature conditions during most of the year, as well as low amounts of available nitrogen, phosphorus, and organic carbon. -

Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis
International Journal of Molecular Sciences Review Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis David Johane Machate 1, Priscila Silva Figueiredo 2 , Gabriela Marcelino 2 , Rita de Cássia Avellaneda Guimarães 2,*, Priscila Aiko Hiane 2 , Danielle Bogo 2, Verônica Assalin Zorgetto Pinheiro 2, Lincoln Carlos Silva de Oliveira 3 and Arnildo Pott 1 1 Graduate Program in Biotechnology and Biodiversity in the Central-West Region of Brazil, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; [email protected] (D.J.M.); [email protected] (A.P.) 2 Graduate Program in Health and Development in the Central-West Region of Brazil, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; pri.fi[email protected] (P.S.F.); [email protected] (G.M.); [email protected] (P.A.H.); [email protected] (D.B.); [email protected] (V.A.Z.P.) 3 Chemistry Institute, Federal University of Mato Grosso do Sul, Campo Grande 79079-900, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-67-3345-7416 Received: 9 March 2020; Accepted: 27 March 2020; Published: 8 June 2020 Abstract: Long-term high-fat dietary intake plays a crucial role in the composition of gut microbiota in animal models and human subjects, which affect directly short-chain fatty acid (SCFA) production and host health. This review aims to highlight the interplay of fatty acid (FA) intake and gut microbiota composition and its interaction with hosts in health promotion and obesity prevention and its related metabolic dysbiosis. -

WO 2018/064165 A2 (.Pdf)
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/064165 A2 05 April 2018 (05.04.2018) W !P O PCT (51) International Patent Classification: Published: A61K 35/74 (20 15.0 1) C12N 1/21 (2006 .01) — without international search report and to be republished (21) International Application Number: upon receipt of that report (Rule 48.2(g)) PCT/US2017/053717 — with sequence listing part of description (Rule 5.2(a)) (22) International Filing Date: 27 September 2017 (27.09.2017) (25) Filing Language: English (26) Publication Langi English (30) Priority Data: 62/400,372 27 September 2016 (27.09.2016) US 62/508,885 19 May 2017 (19.05.2017) US 62/557,566 12 September 2017 (12.09.2017) US (71) Applicant: BOARD OF REGENTS, THE UNIVERSI¬ TY OF TEXAS SYSTEM [US/US]; 210 West 7th St., Austin, TX 78701 (US). (72) Inventors: WARGO, Jennifer; 1814 Bissonnet St., Hous ton, TX 77005 (US). GOPALAKRISHNAN, Vanch- eswaran; 7900 Cambridge, Apt. 10-lb, Houston, TX 77054 (US). (74) Agent: BYRD, Marshall, P.; Parker Highlander PLLC, 1120 S. Capital Of Texas Highway, Bldg. One, Suite 200, Austin, TX 78746 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Table S4. Phylogenetic Distribution of Bacterial and Archaea Genomes in Groups A, B, C, D, and X
Table S4. Phylogenetic distribution of bacterial and archaea genomes in groups A, B, C, D, and X. Group A a: Total number of genomes in the taxon b: Number of group A genomes in the taxon c: Percentage of group A genomes in the taxon a b c cellular organisms 5007 2974 59.4 |__ Bacteria 4769 2935 61.5 | |__ Proteobacteria 1854 1570 84.7 | | |__ Gammaproteobacteria 711 631 88.7 | | | |__ Enterobacterales 112 97 86.6 | | | | |__ Enterobacteriaceae 41 32 78.0 | | | | | |__ unclassified Enterobacteriaceae 13 7 53.8 | | | | |__ Erwiniaceae 30 28 93.3 | | | | | |__ Erwinia 10 10 100.0 | | | | | |__ Buchnera 8 8 100.0 | | | | | | |__ Buchnera aphidicola 8 8 100.0 | | | | | |__ Pantoea 8 8 100.0 | | | | |__ Yersiniaceae 14 14 100.0 | | | | | |__ Serratia 8 8 100.0 | | | | |__ Morganellaceae 13 10 76.9 | | | | |__ Pectobacteriaceae 8 8 100.0 | | | |__ Alteromonadales 94 94 100.0 | | | | |__ Alteromonadaceae 34 34 100.0 | | | | | |__ Marinobacter 12 12 100.0 | | | | |__ Shewanellaceae 17 17 100.0 | | | | | |__ Shewanella 17 17 100.0 | | | | |__ Pseudoalteromonadaceae 16 16 100.0 | | | | | |__ Pseudoalteromonas 15 15 100.0 | | | | |__ Idiomarinaceae 9 9 100.0 | | | | | |__ Idiomarina 9 9 100.0 | | | | |__ Colwelliaceae 6 6 100.0 | | | |__ Pseudomonadales 81 81 100.0 | | | | |__ Moraxellaceae 41 41 100.0 | | | | | |__ Acinetobacter 25 25 100.0 | | | | | |__ Psychrobacter 8 8 100.0 | | | | | |__ Moraxella 6 6 100.0 | | | | |__ Pseudomonadaceae 40 40 100.0 | | | | | |__ Pseudomonas 38 38 100.0 | | | |__ Oceanospirillales 73 72 98.6 | | | | |__ Oceanospirillaceae -

Extensive Microbial Diversity Within the Chicken Gut Microbiome Revealed by Metagenomics and Culture
Extensive microbial diversity within the chicken gut microbiome revealed by metagenomics and culture Rachel Gilroy1, Anuradha Ravi1, Maria Getino2, Isabella Pursley2, Daniel L. Horton2, Nabil-Fareed Alikhan1, Dave Baker1, Karim Gharbi3, Neil Hall3,4, Mick Watson5, Evelien M. Adriaenssens1, Ebenezer Foster-Nyarko1, Sheikh Jarju6, Arss Secka7, Martin Antonio6, Aharon Oren8, Roy R. Chaudhuri9, Roberto La Ragione2, Falk Hildebrand1,3 and Mark J. Pallen1,2,4 1 Quadram Institute Bioscience, Norwich, UK 2 School of Veterinary Medicine, University of Surrey, Guildford, UK 3 Earlham Institute, Norwich Research Park, Norwich, UK 4 University of East Anglia, Norwich, UK 5 Roslin Institute, University of Edinburgh, Edinburgh, UK 6 Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Atlantic Boulevard, Banjul, The Gambia 7 West Africa Livestock Innovation Centre, Banjul, The Gambia 8 Department of Plant and Environmental Sciences, The Alexander Silberman Institute of Life Sciences, Edmond J. Safra Campus, Hebrew University of Jerusalem, Jerusalem, Israel 9 Department of Molecular Biology and Biotechnology, University of Sheffield, Sheffield, UK ABSTRACT Background: The chicken is the most abundant food animal in the world. However, despite its importance, the chicken gut microbiome remains largely undefined. Here, we exploit culture-independent and culture-dependent approaches to reveal extensive taxonomic diversity within this complex microbial community. Results: We performed metagenomic sequencing of fifty chicken faecal samples from Submitted 4 December 2020 two breeds and analysed these, alongside all (n = 582) relevant publicly available Accepted 22 January 2021 chicken metagenomes, to cluster over 20 million non-redundant genes and to Published 6 April 2021 construct over 5,500 metagenome-assembled bacterial genomes. -

Pathogen Challenge and Dietary Shift Alter Microbiota Composition And
fmicb-12-703421 July 19, 2021 Time: 11:40 # 1 ORIGINAL RESEARCH published: 19 July 2021 doi: 10.3389/fmicb.2021.703421 Pathogen Challenge and Dietary Shift Alter Microbiota Composition and Activity in a Mucin-Associated in vitro Model of the Piglet Colon (MPigut-IVM) Simulating Weaning Transition Raphaële Gresse1,2, Frédérique Chaucheyras-Durand1,2, Juan J. Garrido3, Sylvain Denis1, Angeles Jiménez-Marín3, Martin Beaumont4, Tom Van de Wiele5, Evelyne Forano1 and Stéphanie Blanquet-Diot1* 1 INRAE, UMR 454 MEDIS, Université Clermont Auvergne, Clermont-Ferrand, France, 2 Lallemand SAS, Blagnac, France, 3 Grupo de Genómica y Mejora Animal, Departamento de Genética, Facultad de Veterinaria, Universidad de Córdoba, Córdoba, Spain, 4 GenPhySE, INRAE, ENVT, Université de Toulouse, Castanet-Tolosan, France, 5 Center for Microbial Ecology and Technology, Ghent University, Ghent, Belgium Edited by: Wakako Ikeda-Ohtsubo, Tohoku University, Japan Enterotoxigenic Escherichia coli (ETEC) is the principal pathogen responsible for post- Reviewed by: weaning diarrhea in newly weaned piglets. Expansion of ETEC at weaning is thought to Katie Lynn Summers, be the consequence of various stress factors such as transient anorexia, dietary change United States Department or increase in intestinal inflammation and permeability, but the exact mechanisms remain of Agriculture (USDA), United States Åsa Sjöling, to be elucidated. As the use of animal experiments raise more and more ethical Karolinska Institutet (KI), Sweden concerns, we used a recently developed in vitro model of piglet colonic microbiome *Correspondence: and mucobiome, the MPigut-IVM, to evaluate the effects of a simulated weaning Stéphanie Blanquet-Diot [email protected] transition and pathogen challenge at weaning. -

Table S5. the Information of the Bacteria Annotated in the Soil Community at Species Level
Table S5. The information of the bacteria annotated in the soil community at species level No. Phylum Class Order Family Genus Species The number of contigs Abundance(%) 1 Firmicutes Bacilli Bacillales Bacillaceae Bacillus Bacillus cereus 1749 5.145782459 2 Bacteroidetes Cytophagia Cytophagales Hymenobacteraceae Hymenobacter Hymenobacter sedentarius 1538 4.52499338 3 Gemmatimonadetes Gemmatimonadetes Gemmatimonadales Gemmatimonadaceae Gemmatirosa Gemmatirosa kalamazoonesis 1020 3.000970902 4 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas indica 797 2.344876284 5 Firmicutes Bacilli Lactobacillales Streptococcaceae Lactococcus Lactococcus piscium 542 1.594633558 6 Actinobacteria Thermoleophilia Solirubrobacterales Conexibacteraceae Conexibacter Conexibacter woesei 471 1.385742446 7 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas taxi 430 1.265115184 8 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas wittichii 388 1.141545794 9 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas sp. FARSPH 298 0.876754244 10 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sorangium cellulosum 260 0.764953367 11 Proteobacteria Deltaproteobacteria Myxococcales Polyangiaceae Sorangium Sphingomonas sp. Cra20 260 0.764953367 12 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas panacis 252 0.741416341 -

Role of Actinobacteria and Coriobacteriia in the Antidepressant Effects of Ketamine in an Inflammation Model of Depression
Pharmacology, Biochemistry and Behavior 176 (2019) 93–100 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression T Niannian Huanga,1, Dongyu Huaa,1, Gaofeng Zhana, Shan Lia, Bin Zhub, Riyue Jiangb, Ling Yangb, ⁎ ⁎ Jiangjiang Bia, Hui Xua, Kenji Hashimotoc, Ailin Luoa, , Chun Yanga, a Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China b Department of Internal Medicine, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China c Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba 260-8670, Japan ARTICLE INFO ABSTRACT Keywords: Ketamine, an N-methyl-D-aspartic acid receptor (NMDAR) antagonist, elicits rapid-acting and sustained anti- Ketamine depressant effects in treatment-resistant depressed patients. Accumulating evidence suggests that gut microbiota Depression via the gut-brain axis play a role in the pathogenesis of depression, thereby contributing to the antidepressant Lipopolysaccharide actions of certain compounds. Here we investigated the role of gut microbiota in the antidepressant effects of Gut microbiota ketamine in lipopolysaccharide (LPS)-induced inflammation model of depression. Ketamine (10 mg/kg) sig- nificantly attenuated the increased immobility time in forced swimming test (FST), which was associated with the improvements in α-diversity, consisting of Shannon, Simpson and Chao 1 indices. In addition to α-diversity, β-diversity, such as principal coordinates analysis (PCoA), and linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe), showed a differential profile after ketamine treatment. -

Early-Life Gut Dysbiosis Linked to Juvenile Mortality in Ostriches Elin Videvall1,2* , Se Jin Song3,4, Hanna M
Videvall et al. Microbiome (2020) 8:147 https://doi.org/10.1186/s40168-020-00925-7 RESEARCH Open Access Early-life gut dysbiosis linked to juvenile mortality in ostriches Elin Videvall1,2* , Se Jin Song3,4, Hanna M. Bensch1, Maria Strandh1, Anel Engelbrecht5, Naomi Serfontein6, Olof Hellgren1, Adriaan Olivier7, Schalk Cloete5,8, Rob Knight3,4,9,10 and Charlie K. Cornwallis1 Abstract Background: Imbalances in the gut microbial community (dysbiosis) of vertebrates have been associated with several gastrointestinal and autoimmune diseases. However, it is unclear which taxa are associated with gut dysbiosis, and if particular gut regions or specific time periods during ontogeny are more susceptible. We also know very little of this process in non-model organisms, despite an increasing realization of the general importance of gut microbiota for health. Methods: Here, we examine the changes that occur in the microbiome during dysbiosis in different parts of the gastrointestinal tract in a long-lived bird with high juvenile mortality, the ostrich (Struthio camelus). We evaluated the 16S rRNA gene composition of the ileum, cecum, and colon of 68 individuals that died of suspected enterocolitis during the first 3 months of life (diseased individuals), and of 50 healthy individuals that were euthanized as age-matched controls. We combined these data with longitudinal environmental and fecal sampling to identify potential sources of pathogenic bacteria and to unravel at which stage of development dysbiosis- associated bacteria emerge. Results: Diseased individuals had drastically lower microbial alpha diversity and differed substantially in their microbial beta diversity from control individuals in all three regions of the gastrointestinal tract. -

Insights Into the Role of Erysipelotrichaceae in the Human Host
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector OPINION published: 20 November 2015 doi: 10.3389/fcimb.2015.00084 Insights into the Role of Erysipelotrichaceae in the Human Host Nadeem O. Kaakoush * School of Biotechnology and Biomolecular Sciences, Faculty of Science, University of New South Wales, Sydney, NSW, Australia Keywords: Erysipelotrichaceae, gastrointestinal, microbiota, metabolic disorders, inflammatory bowel diseases, colorectal cancer Understanding the human gut microbiota has garnered interest from researchers and clinicians to pharmaceutical companies looking at novel mechanisms to manipulate the microbiota for the benefit of the host. Studies on the gut microbiota can be loosely characterized into three areas that include investigating the microbiota’s role in the physiology of the healthy gut, in the establishment of gastrointestinal disease, and in extra-intestinal manifestations. With deep sequencing technologies now in routine use in the research environment, novel members of the gut microbiota are coming to light, and our understanding of this complex ecosystem and its relationship to the host is slowly improving. IMPORTANCE OF ERYSIPELOTRICHACEAE IN HUMANS Edited by: Reports documenting a potential role for the bacterial family Erysipelotrichaceae, which belongs D. Scott Merrell, to the Firmicutes phylum, in host physiology and/or disease are on the rise. However, more often Uniformed Services University of the than not, these organisms are mentioned in passing, despite the fact that members of this bacterial Health Sciences, USA family appear to be highly immunogenic and can potentially flourish post-treatment with broad Reviewed by: spectrum antibiotics (Zhao et al., 2013; Palm et al., 2014; Dinh et al., 2015). -
Supplementary Data TMAO Microbiome R1
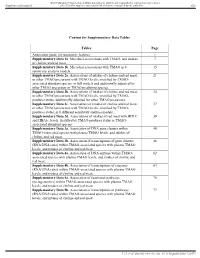
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Dose-Responses Relationship in Glucose Lowering and Gut
microorganisms Article Dose-Responses Relationship in Glucose Lowering and Gut Dysbiosis to Saskatoon Berry Powder Supplementation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice Ruozhi Zhao 1, Fei Huang 1 and Garry X. Shen 1,2,* 1 Department of Internal Medicine, University of Manitoba, Winnipeg, MB R3E 3P4, Canada; [email protected] (R.Z.); [email protected] (F.H.) 2 Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, MB R3E 3P4, Canada * Correspondence: [email protected]; Tel.: +1-204-789-3816; Fax: +1-204-789-3987 Abstract: Administration of freeze-dried powder of Saskatoon berry (SB), a popular fruit enriched with antioxidants, reduced glucose level, inflammatory markers and gut microbiota disorder in high fat-high sucrose (HFHS) diet-induced insulin resistant mice. The present study examined the dose-response relationship in metabolic, inflammatory and gut microbiotic variables to SB power (SBp) supplementation in HFHS diet-fed mice. Male C57 BL/6J mice were fed with HFHS diet supplemented with 0, 1%, 2.5% or 5% SBp for 11 weeks. HFHS diet significantly increased the levels of fast plasma glucose (FPG), cholesterol, triglycerides, insulin, homeostatic model assessment of insulin resistance (HOMA-IR), tumor necrosis factor-α, monocyte chemotactic protein-1 and plasminogen activator inhibitor-1, but decreased fecal Bacteroidetes phylum bacteria and Muribaculaceae family bacteria compared to low fat diet. SBp dose-dependently reduced metabolic and inflammatory Citation: Zhao, R.; Huang, F.; Shen, variables and gut dysbiosis in mice compared with mice receiving HFHS diet alone. Significant G.X. Dose-Responses Relationship in ≥ Glucose Lowering and Gut Dysbiosis attenuation of HFHS diet-induced biochemical disorders were detected in mice receiving 1% SBp.