Bycatch Weight, Composition and Preliminary Estimates of the Impact
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Combined Effects of Urbanization and Connectivity on Iconic Coastal Fishes
Diversity and Distributions, (Diversity Distrib.) (2016) 22, 1328–1341 BIODIVERSITY Combined effects of urbanization and RESEARCH connectivity on iconic coastal fishes Elena Vargas-Fonseca1, Andrew D. Olds1*, Ben L. Gilby1, Rod M. Connolly2, David S. Schoeman1, Chantal M. Huijbers1,2, Glenn A. Hyndes3 and Thomas A. Schlacher1 1School of Science and Engineering, ABSTRACT University of the Sunshine Coast, Aim Disturbance and connectivity shape the structure and spatial distribution Maroochydore DC, Qld 4558, Australia, 2 of animal populations in all ecosystems, but the combined effects of these fac- Australian Rivers Institute – Coast & Estuaries, School of Environment, Griffith tors are rarely measured in coastal seascapes. We used surf zones of exposed University, Gold Coast, Qld 4222, Australia, sandy beaches in eastern Australia as a model seascape to test for combined 3Centre for Marine Ecosystems Research, effects of coastal urbanization and seascape connectivity (i.e. spatial links School of Natural Sciences, Edith Cowan between surf zones, estuaries and rocky headlands) on fish assemblages. University, Perth, WA 6027, Australia Location Four hundred kilometres of exposed surf beaches along the eastern coastline of Australia. A Journal of Conservation Biogeography Methods Fish assemblages were surveyed from surf zones of 14 ocean-exposed sandy beaches using purpose-built surf baited remote underwater video sta- tions. Results The degree of coastal urbanization and connectivity were strongly cor- related with the spatial distribution of fish species richness and abundance and were of greater importance to surf fishes than local surf conditions. Urbaniza- tion was associated with reductions in the abundance of harvested piscivores and fish species richness. Piscivore abundance and species richness were lowest on highly urbanized coastlines, and adjacent to beaches in wilderness areas where recreational fishing is intense. -

Acanthobothrium Urolophi Sp. N., a Tetraphyllidean Cestode (Oncobothriidae) from an Australian Stingaree
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1973 Acanthobothrium urolophi sp. n., a Tetraphyllidean Cestode (Oncobothriidae) from an Australian Stingaree Gerald D. Schmidt University of Northern Colorado Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Schmidt, Gerald D., "Acanthobothrium urolophi sp. n., a Tetraphyllidean Cestode (Oncobothriidae) from an Australian Stingaree" (1973). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 693. https://digitalcommons.unl.edu/parasitologyfacpubs/693 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Schmidt in Proceedings of the Helminthological Society of Washington (1973) 40. Copyright 1973, Helminthological Society of Washington. Used by permission. Acanthobothrium urolophi sp. n., a Tetraphyllidean Cestode (Oncobothriidae) from an Australian Stingaree GERALD D. SCHMIDT Deparbnent of Biology, University of Northern Colorado, Greeley, Colorado 80631 ABSTRACT: Acanthobothrium urolophi sp. n. is described from a common stingaree, Urolophus testa cet/s, from South Australia. It differs from all other species in being apolytic and acraspedote, in having hooks 105-115 fJ- long, one accessory sucker 80-90 wide on each bothridinm, and 40-72 testes in two longitudinal rows. This report is based upon four specimens on anterior end of each bothridium, each with recovered from the spiral valve of a common single accessory sucker 80 to 90 wide. -

16. Jetties, Shipwrecks and Other Artificial Reefs
Jetties, shipwrecks and other artificial reefs. Chapter 16 in: Baker, J.L. (2015) Marine Assets of Yorke Peninsula. Report for Natural Resources - Northern and Yorke / NY NRM Board, South Australia. 16. Jetties, Shipwrecks and Other Artificial Reefs Edithburgh Kleins Point © D. Kinasz © J. Zhang Asset Jetties, Shipwrecks and other Artificial Reefs Description Structures of wood, iron, steel, and other materials, throughout the NY NRM region, ranging from oceanographically exposed through to sheltered locations. Jetties and shipwrecks function as surfaces for attachment of marine plants and attached invertebrates; sheltering and feeding areas for fishes, sharks, rays and invertebrates; and as “fish-attracting” devices, periodically visited by schooling fishes which are attracted to vertical structure. Surrounding sea floor varies according to the location of the jetty or wreck, and includes reef, seagrass, sand, and rubble. There are also two purpose-built artificial reefs in the NY NRM region, constructed of tetrahedon module units, made up vehicle tyres. Main Species Sponges sponges (numerous species, in genera Dysidea, Euryspongia, Darwinella, Aplysilla, Dendrilla, Clathrina and many others) Ascidians / Sea Squirts Red-mouthed Ascidian, Obese Ascidian, and other solitary ascidians / sea squirts Brain Ascidian, and other colonial ascidians Spongy Compound, Leach’s Compound & other compound ascidians Corals gorgonian corals such as Mopsella zimmeri (on current-exposed jetties) soft corals, such as Carijoa (also Drifa sp. on current-exposed jetties) solitary coral Scolymia Bryozoans various species, including various species in Cellaporaria (such as Orange Plate Bryozoan and Nipple Bryozoan) and species in Triphyllozoon (Lace Bryozoans) Gastropod Shells Cowries, Cartrut shell, Triton shells Bivalve Shells Doughboy Scallop, Razorfish Shell, juvenile Native Oyster Jetties, shipwrecks and other artificial reefs. -

Life-History Characteristics of the Eastern Shovelnose Ray, Aptychotrema Rostrata (Shaw, 1794), from Southern Queensland, Australia
CSIRO PUBLISHING Marine and Freshwater Research, 2021, 72, 1280–1289 https://doi.org/10.1071/MF20347 Life-history characteristics of the eastern shovelnose ray, Aptychotrema rostrata (Shaw, 1794), from southern Queensland, Australia Matthew J. Campbell A,B,C, Mark F. McLennanA, Anthony J. CourtneyA and Colin A. SimpfendorferB AQueensland Department of Agriculture and Fisheries, Agri-Science Queensland, Ecosciences Precinct, GPO Box 267, Brisbane, Qld 4001, Australia. BCentre for Sustainable Tropical Fisheries and Aquaculture and College of Science and Engineering, James Cook University, 1 James Cook Drive, Townsville, Qld 4811, Australia. CCorresponding author. Email: [email protected] Abstract. The eastern shovelnose ray (Aptychotrema rostrata) is a medium-sized coastal batoid endemic to the eastern coast of Australia. It is the most common elasmobranch incidentally caught in the Queensland east coast otter trawl fishery, Australia’s largest penaeid-trawl fishery. Despite this, age and growth studies on this species are lacking. The present study estimated the growth parameters and age-at-maturity for A. rostrata on the basis of sampling conducted in southern Queensland, Australia. This study showed that A. rostrata exhibits slow growth and late maturity, which are common life- history strategies among elasmobranchs. Length-at-age data were analysed within a Bayesian framework and the von Bertalanffy growth function (VBGF) best described these data. The growth parameters were estimated as L0 ¼ 193 mm À1 TL, k ¼ 0.08 year and LN ¼ 924 mm TL. Age-at-maturity was found to be 13.3 years and 10.0 years for females and males respectively. The under-sampling of larger, older individuals was overcome by using informative priors, reducing bias in the growth and maturity estimates. -

An Overview of the Elasmobranch By-Catch of the Queensland East Coast Trawl Fishery (Australia) (Elasmobranch Fisheries – Oral)
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N4718 NAFO SCR Doc. 02/97 SCIENTIFIC COUNCIL MEETING – SEPTEMBER 2002 An Overview of the Elasmobranch By-catch of the Queensland East Coast Trawl Fishery (Australia) (Elasmobranch Fisheries – Oral) by P. M. Kynea, A.J. Courtneyb, M.J. Campbellb, K.E. Chilcottb, S.W. Gaddesb, C.T. Turnbullc, C.C. Van Der Geestc and M. B. Bennetta a Department of Anatomy and Developmental Biology, The University of Queensland, Brisbane, 4072, Queensland, Australia b Southern Fisheries Centre, Queensland Department of Primary Industries, PO Box 76, Deception Bay, 4508, Queensland, Australia c Northern Fisheries Centre, Queensland Department of Primary Industries, PO Box 5396, Cairns, 4870, Queensland, Australia E-mail: [email protected] Abstract The Queensland East Coast Trawl Fishery (ETCF) is a complex multi-species and multi-sector fishery operating along Queensland’s eastern coastline, with combined annual landings of close to 10 000 tons. Elasmobranchs represent a relatively small, but potentially ecologically significant component of by-catch in this fishery. At least 94 species of elasmobranchs occur in the managed area of the ECTF and a study has been initiated to examine elasmobranch by-catch in four sectors of the fishery, as part of a larger Queensland Department of Primary Industries by-catch project. A total of 42 elasmobranch and one holocephalan species have been recorded as by- catch in the fishery. Preliminary results from fishery-independent (FI) surveys indicate that elasmobranch by-catch is highly variable between fishery sectors. Elasmobranch by-catch is extremely low in the tiger/Endeavour prawn sector, low in the eastern king prawn – deep water sector (EKP-D), and moderate in the EKP – shallow water sector (EKP-S). -

Observer-Based Study of Targeted Commercial Fishing for Large Shark Species in Waters Off Northern New South Wales
Observer-based study of targeted commercial fishing for large shark species in waters off northern New South Wales William G. Macbeth, Pascal T. Geraghty, Victor M. Peddemors and Charles A. Gray Industry & Investment NSW Cronulla Fisheries Research Centre of Excellence P.O. Box 21, Cronulla, NSW 2230, Australia Northern Rivers Catchment Management Authority Project No. IS8-9-M-2 November 2009 Industry & Investment NSW – Fisheries Final Report Series No. 114 ISSN 1837-2112 Observer-based study of targeted commercial fishing for large shark species in waters off northern New South Wales November 2009 Authors: Macbeth, W.G., Geraghty, P.T., Peddemors, V.M. and Gray, C.A. Published By: Industry & Investment NSW (now incorporating NSW Department of Primary Industries) Postal Address: Cronulla Fisheries Research Centre of Excellence, PO Box 21, Cronulla, NSW, 2230 Internet: www.industry.nsw.gov.au © Department of Industry and Investment (Industry & Investment NSW) and the Northern Rivers Catchment Management Authority This work is copyright. Except as permitted under the Copyright Act, no part of this reproduction may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Neither may information be stored electronically in any form whatsoever without such permission. DISCLAIMER The publishers do not warrant that the information in this report is free from errors or omissions. The publishers do not accept any form of liability, be it contractual, tortuous or otherwise, for the contents of this report for any consequences arising from its use or any reliance placed on it. The information, opinions and advice contained in this report may not relate to, or be relevant to, a reader’s particular circumstance. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

Mme. Hadjer MAHDI DOCTORAT
REPUBLIQUE ALGERIENNE DEMOCRATIQUE ET POPULAIRE MINISTERE DE L’ENSEIGNEMENT SUPERIEUR ET DE LA RECHERCHE SCIENTIFIQUE UNIVERSITE D’ORAN FACULTE DES SCIENCES DE LA NATURE ET DE LA VIE DEPARTEMENT DE BIOLOGIE THESE Présentée par Mme. Hadjer MAHDI Pour l’obtention du diplôme de DOCTORAT L.M.D EN BIOLOGIE Option : Sciences de la mer et du littoral Intitulée Biologie et écologie du Pageot commun Pagellus erythrinus ( Linnaeus., 1758) de la côte ouest algérienne Soutenue le: devant le jury composé de : PRESIDENT : BOUDERBALA. M PROFESSEUR, UNIVERSITE D’ORAN-1 AHMED BENBELLA EXAMINATRICE : SOUALILI D.L PROFESSEUR, UNIVERSITE DE MOSTAGANEM- ABDELHAMID BENBADIS EXAMINATRICE : DERMECHE.S MAITRE DE CONFERENCE A , UNIVERSITE D’ORAN -1 AHMED BENBELLA EXAMINATEUR : MESLI L. PROFESSEUR, UNIVERSITE DE TLEMCEN, ABOUBAKR BELKAID EXAMINATEUR : ABI-AYAD S.M.E.A. PROFESSEUR, UNIVERSITE D’ORAN -1 AHMED BENBELLA PROMOTEUR : BENSAHLA TALET L. MAITRE DE CONFERENCE A, UNIVERSITE D’ORAN Année universitaire 2017-2018 REMERCIEMENTS Je tiens à exprimer mes plus vifs remerciements au Dr BENSAHLA TALET Lotfi qui fut pour moi un directeur de thèse attentif et disponible malgré ses nombreuses charges, sa compétence, sa rigueur scientifique et son expérience m’ont beaucoup appris. Ils ont été des moteurs de mon travail de chercheur. Je tiens à rendre hommage à notre cher et regretté professeur Zitouni BOUTIBA qui a donné toute son énergie tout au long de sa carrière pour que nous puissions être la aujourd’hui parmi vous. Et qu’il reste une référence pour nous et pour toutes les générations à venir. Monsieur BOUTIBA restera gravé dans notre mémoire à tout jamais. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Download (4MB)
This file is part of the following reference: Cappo, Mike (2010) Development of a baited video technique and spatial models to explain patterns of fish biodiversity in inter-reef waters. PhD thesis, James Cook University. Access to this file is available from: http://eprints.jcu.edu.au/15420 6. SHELF-SCALE PATTERNS OF VERTEBRATE ASSEMBLAGES IN THE INTER-REEFAL WATERS OF THE GREAT BARRIER REEF MARINE PARK 6.1 INTRODUCTION Definition of environmental boundaries in species ranges, species assemblages and species ‘stock structure’ is central to conservation planning, fisheries management and the understanding of ecosystem and evolutionary processes. This is particularly important in the tropics, where marine fisheries exploit co-occurring vertebrates in large assemblages (hundreds of taxa) of ‘target’ and ‘bycatch’ species (Sainsbury et al. 1997). For the purposes of this thesis, an assemblage is defined as the species available (to the BRUVS sampling technique) in the same place at the same time. Ecological analysis of assemblage structure can assist in defining spatial ‘assemblage production units’ for the assignment of particular zones of a fishery to specific sectors, gear types and harvest pressures (Garces et al. 2006b). Such analysis can also be used with knowledge of spatial patterns in fishing effort and vulnerability to capture to assess risks to metapopulations exposed to locally intensive fishing pressure (Pitcher et al. 2000; Astles et al. 2006; Ellis et al. 2008). If the relationship between assemblages and critical features of their habitats (such as substratum type) is known well, then ‘area-based management’ could use maps of major habitat features as a proxy for the populations themselves (Bax & Williams 2001; Anderson et al. -

WCPTOC3.CHP:Corel VENTURA
click for previous page INDEX OF SCIENTIFIC AND VERNACULAR NAMES Explanation of the System Italics : Valid scientific names (genera and species). Italics : Synonyms (genera and species), misidentifications. ROMAN : Family names. ROMAN : Names of divisions, classes, subclasses, orders, suborders, and subfamilies. Roman: FAO and local names. 2040 The Living Marine Resources of the Western Central Pacific A Alepocephalids ...................1895 Alepocephalus agassizii ............1888 abbotti, Notacanthus .............. 1628 Alepocephalus australis ............1888 abbreviata, Harengula .............1796 Alepocephalus bairdii ............. 1888 abbreviatus, Gonorynchus ...........1826 Alepocephalus longiceps ............1888 abei, Chaunax .................. 2020 Alfonsinos ......................1578 abnormis, Ilisha ................. 1758 See also Vol. 4 ACANTHURIDAE ..............1610, 1967 Alicefranche....................1712 See also Vol. 6 Alice taches d’or ..................1709 ACANTHUROIDEI.................1609 Allenbatrachus grunniens ...........2001 See also Vol. 6 Allenbatrachus reticulatus...........2001 Acetes .......................1753 Alosa alburnus .................. 1816 ACROPOMATIDAE ................1584 Alosa brevis ................... 1802 See also Vol. 4 Alosachata.....................1791 acuta, Dussumieria............ 1792-1793 Alosa malayana ................. 1802 acutus, Arius ...................1839 Aloseàgrosyeux..................1763 ADRIANICHTHYIDAE ...............1573 Aloseàmuseaucourt................1791 See -
WCPTOC3.CHP:Corel VENTURA
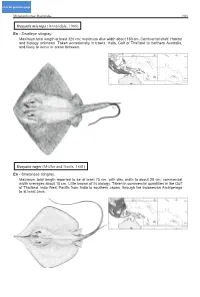
click for previous page Myliobatiformes: Dasyatidae 1501 Dasyatis microps (Annandale, 1908) En - Smalleye stingray. Maximum total length at least 320 cm; maximum disc width about 180 cm. Continental shelf. Habitat and biology unknown. Taken occasionally in trawls. India, Gulf of Thailand to northern Australia, and likely to occur in areas between. Dasyatis zugei (Müller and Henle, 1841) En - Sharpnose stingray. Maximum total length reported to be at least 75 cm, with disc width to about 29 cm; commercial width averages about 18 cm. Little known of its biology. Taken in commercial quantities in the Gulf of Thailand. Indo-West Pacific from India to southern Japan, through the Indonesian Archipelago to at least Java. 1502 Batoid Fishes Himantura imbricata (Bloch and Schneider, 1801) En - Scaly whipray. Maximum total length about 65 cm; maximum disc width 22 cm. Distribution through Indo-Malay Archipelago not well defined (regularly confused with Himantura walga). Thought to occur from the Red Sea to Java. Himantura marginata (Blyth, 1860) En - Blackedge whipray. Maximum total length at least 345 cm; maximum disc width 179 cm. Poorly known species. Thought to occur off India, Sri Lanka, and Myanmar, may venture into Indonesian waters; possibly off Mozambique. Myliobatiformes: Dasyatidae 1503 Himantura oxyrhynchus (Sauvage, 1878) En - Marbled whipray. Maximum total length at least 126 cm; maximum disc width 36 cm. A fresh-water species; can also be found in estuaries. Unknown commercial use. Cambodia, Thailand, and Borneo. Himantura signifer Compagno and Roberts, 1982 En - Pale whipray. Maximum total length at least 178 cm; maximum disc width 38 cm. A fresh-water species; can also be found in estuaries.