Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Terrestrial Ecological Classifications
INTERNATIONAL ECOLOGICAL CLASSIFICATION STANDARD: TERRESTRIAL ECOLOGICAL CLASSIFICATIONS Alliances and Groups of the Central Basin and Range Ecoregion Appendix Accompanying Report and Field Keys for the Central Basin and Range Ecoregion: NatureServe_2017_NVC Field Keys and Report_Nov_2017_CBR.pdf 1 December 2017 by NatureServe 600 North Fairfax Drive, 7th Floor Arlington, VA 22203 1680 38th St., Suite 120 Boulder, CO 80301 This subset of the International Ecological Classification Standard covers vegetation alliances and groups of the Central Basin and Range Ecoregion. This classification has been developed in consultation with many individuals and agencies and incorporates information from a variety of publications and other classifications. Comments and suggestions regarding the contents of this subset should be directed to Mary J. Russo, Central Ecology Data Manager, NC <[email protected]> and Marion Reid, Senior Regional Ecologist, Boulder, CO <[email protected]>. Copyright © 2017 NatureServe, 4600 North Fairfax Drive, 7th floor Arlington, VA 22203, U.S.A. All Rights Reserved. Citations: The following citation should be used in any published materials which reference ecological system and/or International Vegetation Classification (IVC hierarchy) and association data: NatureServe. 2017. International Ecological Classification Standard: Terrestrial Ecological Classifications. NatureServe Central Databases. Arlington, VA. U.S.A. Data current as of 1 December 2017. Restrictions on Use: Permission to use, copy and distribute these data is hereby granted under the following conditions: 1. The above copyright notice must appear in all documents and reports; 2. Any use must be for informational purposes only and in no instance for commercial purposes; 3. Some data may be altered in format for analytical purposes, however the data should still be referenced using the citation above. -

Willamette Valley Oak and Prairie Cooperative Strategic Action Plan
Willamette Valley Oak and Prairie Cooperative Strategic Action Plan March 2020 Willamette Valley Oak and Prairie Cooperative Strategic Action Plan, March 2020 Page i Acknowledgements Steering Committee: Clinton Begley Long Tom Watershed Council Sara Evans-Peters Pacific Birds Habitat Joint Venture Claire Fiegener Greenbelt Land Trust Tom Kaye Institute for Applied Ecology Nicole Maness Willamette Partnership Shelly Miller City of Eugene Will Neuhauser Yamhill Partners for Land and Water Michael Pope Greenbelt Land Trust Lawrence Schwabe Confederated Tribes Grand Ronde Bruce Taylor Pacific Birds Habitat Joint Venture Stan van de Wetering Confederated Tribes Siletz Kelly Warren Ducks Unlimited INC Contractors: Jeff Krueger JK Environments Carolyn Menke Institute for Applied Ecology Working Group: Bob Altman American Bird Conservancy Ed Alverson Lane County Parks Marc Bell Polk Soil and Water Conservation District Andrea Berkley Oregon Parks and Recreation Department Matt Blakeley-Smith Greenbelt Land Trust Jason Blazar Friends of Buford Park & Mt. Pisgah Lynda Boyer Heritage Seedlings Joe Buttafuoco The Nature Conservancy Mikki Collins U.S. Fish & Wildlife Service Sarah Deumling Zena Forest Daniel Dietz McKenzie River Trust Sarah Dyrdhal Middle Fork Willamette Watershed Council Matt Gibbons The Nature Conservancy Lauren Grand Oregon State University Extension Service Jarod Jebousek U.S. Fish & Wildlife Service Bart Johnson University of Oregon Pat Johnston U.S Bureau of Land Management Molly Juillerat U.S. National Forest Service (MFWRD) Cameron King U.S. Fish & Wildlife Service John Klock U.S Bureau of Land Management Ann Kreager Oregon Department of Fish and Wildlife Amie Loop-Frison Yamhill Soil and Water Conservation District Katie MacKendrick Long Tom Watershed Council Anne Mary Meyers Oregon Department of Fish and Wildlife Willamette Valley Oak and Prairie Cooperative Strategic Action Plan, March 2020 Page ii Mark Miller Trout Mountain Forestry Kevin O'Hara U.S. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Disturbance Macroecology: An Information Entropy Approach for Cross-System Comparisons of Ecosystems in Transition Permalink https://escholarship.org/uc/item/7rd5d4hv Author Newman, Erica A. Publication Date 2016 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Disturbance Macroecology: An Information Entropy Approach for Cross-System Comparisons of Ecosystems in Transition by Erica Anna Newman A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in the Energy and Resources Group in the Graduate Division of the University of California, Berkeley Committee in charge: Professor John Harte, Co-Chair Professor Max Alan Moritz, Co-Chair Professor Steven R. Beissinger Professor Scott L. Stephens Spring 2016 Abstract Disturbance Macroecology: An Information Entropy Approach for Cross-System Comparisons of Ecosystems in Transition by Erica Anna Newman Doctor of Philosophy in Energy and Resources University of California, Berkeley Professor John Harte, Co-Chair Professor Max Alan Moritz, Co-Chair Little is known about how metrics of biodiversity and abundance scale in ecologically disturbed and disrupted systems. Natural disturbances have a fundamental role in structuring ecological communities, and the study of these processes and extension to novel ecological disruptions is of increasing importance due to global change and mounting human impacts. Numerous studies have demonstrated the importance of natural disturbance in determining basic ecological properties of an ecosystem, including species diversity, membership, and relative abundances of those species, as well as overall productivity. Although estimating ecological metrics at both the species and community level is of critical importance to conservation goals, predicting the impacts of disturbance and disruption, including anthropogenic changes, on ecosystems is a major problem for ecological theory for several reasons. -

Bruce Newhouse Is the Owner-Operator of Salix Associates
Salix Associates 2525 Potter, Eugene, OR 97405 ◦ tele 541.343.2364 Salix Associates salixassociates.com …offers services in ecologically-based natural resources planning, including botanical/biodiversity surveying, wildlife habitat inventory and analysis, restoration and management planning, and related environmental planning tasks and issues. The Salix Associates work philosophy emphasizes honesty, accuracy, creativity, thoroughness and scientific credibility with a strong interest in continuing education and advancing field and office skills. Work should be enjoyable, and I strive to make it so. Bruce Newhouse is the owner-operator of Salix Associates. He is a field ecologist, botanist and environmental planner specializing in ecology, botany, ornithology, lepidoptery and mycology. His work includes habitat inventory, analysis, planning, restoration and management. He has a B.S. from Oregon State University in environmental science, and worked for 10 years as a county and city land use planner specializing in natural resources before becoming a private consultant in 1989. As a consultant, he has contracted with federal, state and local public and private agencies and landowners for rare and invasive plant surveys and mapping, comprehensive and integrated natural resource inventories, restoration and management planning, environmental planning and special natural resource projects such as butterfly host plant analysis. He also is an experienced science field and classroom instructor (University of Oregon, Oregon State University, Portland State University, Lane Community College, et al.) specializing in the identification of sedges, rushes, grasses, and more generally, rare, native and invasive plant species, butterflies and fungi, and is a volunteer ecological advisor to several nonprofit groups and committees in the greater Eugene area. -

Plant Species Table
Appendix D Plant Species Table Plant Species Table Listing Status Scientific Name Federal/ Blooming Common Name State/CRPR Habitat and Elevation Period Potential On-Site Occurrence Survey Results Astragalus umbraticus 2B.3 Cismontane woodland, lower montane May-August Unlikely. Few of the habitat components meeting Not Present Bald Mountain Milk-vetch coniferous forest. Dry open oak and the species requirements are present, and/or the pine woodlands; sometimes on majority of habitat on and adjacent to the site is roadsides. 150-1250 m. unsuitable or of very poor quality. Bensoniella oregona SR/1B.1 Bogs and fens, lower montane May-July Absent. Suitable habitat is not present in the study Not Present Bensoniella coniferous forest, meadows and seeps. area. Wet meadows and openings in forest. 915-1400 m. Botrypus virginianus 2B.2 Bogs and fens, lower montane June- Absent. Suitable habitat is not present in the study Not Present Rattlesnake fern coniferous forest, meadows and seeps, September area. riparian forest. 715-1355 m. Carex arcta 2B.2 Bogs and fens, north coast coniferous June- Absent. Suitable habitat is not present in the study Not Present Northern clustered sedge forest. Mesic sites. 60-1400 m. September area. Carex praticola 2B.2 Meadows. Moist to wet meadows. 0- May-July Absent. Suitable habitat is not present in the study Not Present Northern meadow sedge 3200m. area. Cornus canadensis 2B.2 North coast coniferous forest, bogs and May-July Absent. Suitable habitat is not present in the study Not Present Bunchberry fens, meadows and seeps. 60-1920 m. area. Epilobium oreganum 1B.2 Bogs and fens, lower montane June- Unlikely. -

California Status Factors
California Status Factors Elcode PDASTEC0A0 Gname EUCEPHALUS VIALIS Gcomname Wayside aster Number of Occurrences A = 1 - 5 Comments One occurrence known in Del Norte County, California. More sites may exist in the Siskiyou Mountains than has been currently reported. Number of Occurrences with Good Viability U = Unknown what number of occurrences with good viability Comments There is no information for the one record. Population Size A = 1-50 individuals Comments There is no information for the one record. Basing an estimate using Oregon data, most populations for this species are less than 50. Range Extent A = <100 km2 (less than about 40 square miles) Comments One occurrence known in Del Norte County, California, assumed to be less than 100 km2. Area of Occupancy A = <0.4 km2 (less than about 100 acres) LA = <4 km (less than about 2.5 miles) Comments Long-term Trend in Population Size, Extent of Occurrence, Area of Occupancy, and/or Number or Condition of Occurrences E = Relatively Stable (±25% change) Comments This plant seems to tolerate slight disturbance, and may prefer open to semi-shade. Plants growing under closed canopy appear less vigorous and robust. In areas that have human disturbance, roadsides, gardens etc., populations appear to expand and will probably be shaded out. Short-term Trend in Population Size, Extent of Occurrence, Area of Occupancy, and/or Number or Condition of Occurrences E = Stable. Population, range, area occupied, and/or number or condition of occurrences unchanged or remaining within ±10% fluctuation Comments This plant seems to tolerate slight disturbance, and may prefer open to semi-shade. -

Washington Flora Checklist a Checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium
Washington Flora Checklist A checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium The Washington Flora Checklist aims to be a complete list of the native and naturalized vascular plants of Washington State, with current classifications, nomenclature and synonymy. The checklist currently contains 3,929 terminal taxa (species, subspecies, and varieties). Taxa included in the checklist: * Native taxa whether extant, extirpated, or extinct. * Exotic taxa that are naturalized, escaped from cultivation, or persisting wild. * Waifs (e.g., ballast plants, escaped crop plants) and other scarcely collected exotics. * Interspecific hybrids that are frequent or self-maintaining. * Some unnamed taxa in the process of being described. Family classifications follow APG IV for angiosperms, PPG I (J. Syst. Evol. 54:563?603. 2016.) for pteridophytes, and Christenhusz et al. (Phytotaxa 19:55?70. 2011.) for gymnosperms, with a few exceptions. Nomenclature and synonymy at the rank of genus and below follows the 2nd Edition of the Flora of the Pacific Northwest except where superceded by new information. Accepted names are indicated with blue font; synonyms with black font. Native species and infraspecies are marked with boldface font. Please note: This is a working checklist, continuously updated. Use it at your discretion. Created from the Washington Flora Checklist Database on September 17th, 2018 at 9:47pm PST. Available online at http://biology.burke.washington.edu/waflora/checklist.php Comments and questions should be addressed to the checklist administrators: David Giblin ([email protected]) Peter Zika ([email protected]) Suggested citation: Weinmann, F., P.F. Zika, D.E. Giblin, B. -
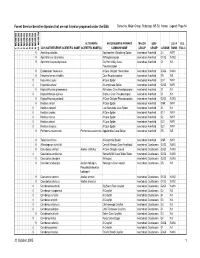
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Rare, Threatened and Endangered Species of Oregon
Portland State University PDXScholar Institute for Natural Resources Publications Institute for Natural Resources - Portland 8-2016 Rare, Threatened and Endangered Species of Oregon James S. Kagan Portland State University Sue Vrilakas Portland State University, [email protected] John A. Christy Portland State University Eleanor P. Gaines Portland State University Lindsey Wise Portland State University See next page for additional authors Follow this and additional works at: https://pdxscholar.library.pdx.edu/naturalresources_pub Part of the Biodiversity Commons, Biology Commons, and the Zoology Commons Let us know how access to this document benefits ou.y Citation Details Oregon Biodiversity Information Center. 2016. Rare, Threatened and Endangered Species of Oregon. Institute for Natural Resources, Portland State University, Portland, Oregon. 130 pp. This Book is brought to you for free and open access. It has been accepted for inclusion in Institute for Natural Resources Publications by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Authors James S. Kagan, Sue Vrilakas, John A. Christy, Eleanor P. Gaines, Lindsey Wise, Cameron Pahl, and Kathy Howell This book is available at PDXScholar: https://pdxscholar.library.pdx.edu/naturalresources_pub/25 RARE, THREATENED AND ENDANGERED SPECIES OF OREGON OREGON BIODIVERSITY INFORMATION CENTER August 2016 Oregon Biodiversity Information Center Institute for Natural Resources Portland State University P.O. Box 751, -

Waterton Lakes National Park • Common Name(Order Family Genus Species)
Waterton Lakes National Park Flora • Common Name(Order Family Genus species) Monocotyledons • Arrow-grass, Marsh (Najadales Juncaginaceae Triglochin palustris) • Arrow-grass, Seaside (Najadales Juncaginaceae Triglochin maritima) • Arrowhead, Northern (Alismatales Alismataceae Sagittaria cuneata) • Asphodel, Sticky False (Liliales Liliaceae Triantha glutinosa) • Barley, Foxtail (Poales Poaceae/Gramineae Hordeum jubatum) • Bear-grass (Liliales Liliaceae Xerophyllum tenax) • Bentgrass, Alpine (Poales Poaceae/Gramineae Podagrostis humilis) • Bentgrass, Creeping (Poales Poaceae/Gramineae Agrostis stolonifera) • Bentgrass, Green (Poales Poaceae/Gramineae Calamagrostis stricta) • Bentgrass, Spike (Poales Poaceae/Gramineae Agrostis exarata) • Bluegrass, Alpine (Poales Poaceae/Gramineae Poa alpina) • Bluegrass, Annual (Poales Poaceae/Gramineae Poa annua) • Bluegrass, Arctic (Poales Poaceae/Gramineae Poa arctica) • Bluegrass, Plains (Poales Poaceae/Gramineae Poa arida) • Bluegrass, Bulbous (Poales Poaceae/Gramineae Poa bulbosa) • Bluegrass, Canada (Poales Poaceae/Gramineae Poa compressa) • Bluegrass, Cusick's (Poales Poaceae/Gramineae Poa cusickii) • Bluegrass, Fendler's (Poales Poaceae/Gramineae Poa fendleriana) • Bluegrass, Glaucous (Poales Poaceae/Gramineae Poa glauca) • Bluegrass, Inland (Poales Poaceae/Gramineae Poa interior) • Bluegrass, Fowl (Poales Poaceae/Gramineae Poa palustris) • Bluegrass, Patterson's (Poales Poaceae/Gramineae Poa pattersonii) • Bluegrass, Kentucky (Poales Poaceae/Gramineae Poa pratensis) • Bluegrass, Sandberg's (Poales -

Appendix 15-A
Appendix 15-A Terrestrial Wildlife and Vegetation Baseline Report HARPER CREEK PROJECT Application for an Environmental Assessment Certificate / Environmental Impact Statement Harper Creek Mine Project Terrestrial Wildlife and Vegetation Baseline Report Prepared for Harper Creek Mining Corp. c/o Yellowhead Mining Inc. 730 – 800 West Pender Street Vancouver, BC V6C 2V6 Prepared by: This image cannot currently be displayed. Keystone Wildlife Research Ltd. 112-9547 152 Street Surrey, BC V3R 5Y5 August 2014 Harper Creek Mine Project Terrestrial Baseline Report DISCLAIMER This report was prepared exclusively for Harper Creek Mining Corporation (HCMC) by Keystone Wildlife Research Ltd. The quality of information, conclusions and estimates contained herein is consistent with the level of effort expended and is based on: i) information on the Project activities, facilities, and workforce available at the time of preparation; ii) data collected by Keystone Wildlife Research Ltd. and its subconsultants, and/or supplied by outside sources; and iii) the assumptions, conditions and qualifications set forth in this report. This report is intended for use by HCMC only, subject to the terms and conditions of its contract with Keystone Wildlife Research Ltd. Any other use or reliance on this report by any third party is at that party’s sole risk. This image cannot currently be displayed. Keystone Wildlife Research Ltd. Page ii Harper Creek Mine Project Terrestrial Baseline Report EXECUTIVE SUMMARY The Harper Creek Project (the Project) is a proposed open pit copper mine located in south- central British Columbia (BC), approximately 150 km northeast by road from Kamloops. The Project has an estimated 28-year mine life based on a process plant throughput of 70,000 tonnes per day. -

Final Environmental Impact Statement: Table of Contents Table of Contents – Volume 2 Changes Between the Draft and Final EIS
BLM Oregon State Office Final Environmental Impact Statement Bureau of Land Management Vegetation Treatments Using Herbicides on BLM Lands in Oregon Volume 2 - Appendices October 2005 October July 2010 FES 10-23 BLM/OR/WA/AE-10/077+1792 As the Nation’s principal conservation agency, the Department of the Interior has responsibility for most of our nationally owned public lands and natural resources. This Cover: Southeast of Richland, Oregon along the Brownlee Reservoir includes fostering the wisest use (Snake River), a rancher views vast stands of medusahead (a noxious weed). of our land and water resources, The area is mixed BLM/private ownership (photographer: Matt Kniesel). protecting our fish and wildlife, preserving the environmental and cultural values of our national parks and historical places, and providing for the enjoyment of life through outdoor recreation. The Department assesses our energy and mineral resources and works to assure that their development is in the best interest of all our people. The Department also has a major responsibility for American Indian reservation communities and for people who live in Island Territories under U.S. administration. Because science cannot, in any practical sense, assure safety through any testing regime, pesticide use should be approached cautiously. (EPA scoping comment, July 28, 2008) Our present technologies for countering invasive non-native weeds are rudimentary and few: control by biological agents, manual eradication, mechanized removal, fire, and herbicides. All have limitations; all are essential (Jake Sigg, California Native Plant Society 1999) Final Environmental Impact Statement: Table of Contents Table of Contents – Volume 2 Changes Between the Draft and Final EIS .