Association Between 1, 5-Anhydroglucitol and Acute C Peptide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
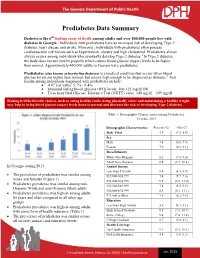
2015 Prediabetes Data Summary
The Georgia Department of Public Health Prediabetes Data Summary Diabetes is the 6th leading cause of death among adults and over 800,000 people live with diabetes in Georgia.1 Individuals with prediabetes have an increased risk of developing Type 2 diabetes, heart disease and stroke. Moreover, individuals with prediabetes often possess cardiovascular risk factors such as hypertension, obesity and high cholesterol. Prediabetes almost always occurs among individuals who eventually develop Type 2 diabetes.2 In Type 2 diabetes, the body does not use insulin properly which causes blood glucose (sugar) levels to be higher than normal. Approximately 450,000 adults in Georgia have prediabetes. Prediabetes (also known as borderline diabetes) is a medical condition that occurs when blood glucose levels are higher than normal, but are not high enough to be diagnosed as diabetes.3 Test results among individuals diagnosed with prediabetes include: A1C test value: 5.7% - 6.4% Impaired fasting blood glucose (IFG) levels: 100-125 mg/dl OR Two- hour Oral Glucose Tolerance Test (OGTT) value: 140 mg/dl – 199 mg/dl Making healt hy lifestyle choices, such as eating healthy foods, being physically active and maintaining a healthy weight, may help to bring blood glucose (sugar) levels down to normal and decrease the risk of developing Type 2 diabetes Table 1. Demographic Characteristics among Prediabetics, Georgia, 2013 Demographic Characteristics Percent (%) 95% CI State Total 7.9 (7.2, 8.9) Sex Male 7.8 (6.8, 9.5) Female 7.9 (6.9, 9.1) Race/Ethnicity -

Decrease of Fructosamine Levels During Treatment with Adalimumab
European Journal of Endocrinology (2007) 156 291–293 ISSN 0804-4643 CASE REPORT Decrease of fructosamine levels during treatment with adalimumab in patients with both diabetes and rheumatoid arthritis I C van Eijk1, M J L Peters1,2, M T Nurmohamed1,2,3, A W van Deutekom4, B A C Dijkmans1,2 and S Simsek4 1Department of Rheumatology, Jan van Breemen Institute, Amsterdam, The Netherlands, 2Department of Rheumatology, VU University Medical Center, Amsterdam, The Netherlands, 3Department of Internal Medicine, VU University Medical Center, Amsterdam, The Netherlands and 4Department of Endocrinology/Diabetes Center, VU University Medical Center, PO Box 7057, 1007 MB, Boelelaan 1117, Amsterdam, The Netherlands (Correspondence should be addressed to S Simsek; Email: [email protected]) Abstract Tumour necrosis factor a (TNFa) is a pro-inflammatory cytokine which has been closely linked to obesity and insulin resistance. We present two cases of patients with rheumatoid arthritis (RA) and concomitant diabetes mellitus, who showed a marked decrease of fructosamine levels after initiating therapy with adalimumab, a TNFa-blocking agent, for active RA. This finding may implicate that TNFa blockade causes better glycaemic control in RA patients with concomitant diabetes, possibly by improving insulin resistance. European Journal of Endocrinology 156 291–293 Introduction Research design and methods Tumour necrosis factor a (TNFa), a pro-inflammatory A patient with known diabetes type 1 and concomitant cytokine, plays an important role in inflammatory and RA showed a marked improvement of HbA1c levels after autoimmune diseases like rheumatoid arthritis (RA). initiation of adalimumab, a recombinant human IgG1- TNFa has also been closely linked to obesity and insulin MAB, therapy for active RA when she visited the resistance (1). -

Comparative Evaluation of Fructosamine and Hba1c As a Marker of Glycemic Control in Type 2 Diabetes: a Hospital Based Study
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Comparative Evaluation of Fructosamine and HbA1c as a Marker of Glycemic Control in Type 2 Diabetes: A Hospital Based Study Dr. Jyoti Goyal1, Dr. Nibhriti Das2, Dr. Navin Kumar3, Ms. Seema Raghav4, Dr. Paramjeet Singh Bhatia5, Dr. Karunesh Prasad Singh6, Dr. Sabari Das7 1DNB, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, 2Ex-Director of Laboratory services and Additional Dean Research and Academics, Nayati Healthcare and Research Centre, Mathura, India- 281003, 3Ph.D, Biostatistitian, Department of Biostatistics, Nayati Healthcare and Research Centre, Mathura, India-281003. 4M.Sc., Certified Diabetes Educator, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 5MD, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 6MD Physician, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 7Department of Laboratory Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, Corresponding Author: Dr. Jyoti Goyal ABSTRACT Introduction: Management of type 2 diabetes revolves around achievement of target glycemic control with the help of antidiabetic drugs or insulin. There are various markers for measurement of glyceamic control like HbA1c, Mean Blood Glucose and fructosamine levels. Though HbA1c is a well validated standard method for assessment of glycemic control but it has also got certain limitations. Fructosamine, a less explored method may be used as an alternative marker for an assessment of glycemic control in cases where HbA1c is unreliable or unavailable. The objective of this study is to compare the fructosamine levels with HbA1c in assessment of glycemic control in type 2 diabetics so as to assess the utility of fructosamine as an alternative marker for evaluation of glucose control. -

Metformin for Prediabetes
FACT SHEET FOR PATIENTS AND FAMILIES Metformin for Prediabetes What is metformin? Metformin [met-FORE-min] is a medication that is often prescribed for prediabetes in order to help prevent type 2 diabetes. It is taken by mouth (orally) as a pill. Like other prediabetes medications, it works best when you follow the rest of your treatment plan, like improving your nutrition by eating more fruits and vegetables, being physically active every day, and in most cases, losing weight. What does it do? Metformin helps lower your blood glucose (blood sugar). It does this by: Does metformin cause hypoglycemia • Decreasing the amount of glucose released by your (low blood glucose)? liver. Less glucose enters into your bloodstream. No. Metformin doesn’t cause hypoglycemia by itself. • Increasing the ability of your muscles to use glucose But combined with other medications, vigorous for energy. As more glucose is used, more glucose exercise, or too little food, it can make your blood leaves your bloodstream. glucose drop too low. Why is metformin important Since low blood glucose can be dangerous, make sure for my health? that you and your family know the symptoms. These include feeling shaky, sweaty, hungry, and irritable. Metformin can’t cure your prediabetes. But, by If you have these symptoms, take some quick-acting helping control your blood glucose, it can help sugar. Good sources are 3 or 4 glucose tablets, a half- prevent type 2 diabetes, especially among those at cup of fruit juice or regular soda, or a tablespoon of highest risk for developing type 2 diabetes. -

Prediabetes: What Is It and What Can I Do?
Prediabetes: What Is It and What Can I Do? What is prediabetes? Weight loss can delay or prevent diabetes. Prediabetes is a condition that comes before Reaching a healthy weight can help you a lot. diabetes. It means your blood glucose levels are If you’re overweight, any weight loss, even higher than normal but aren’t high enough to be 7 percent of your weight (for example, losing called diabetes. about 15 pounds if you weigh 200), may lower your risk for diabetes. There are no clear symptoms of prediabetes. You can have it and not know it. If I have prediabetes, what does it mean? It means you might get type 2 diabetes soon or down the road. You are also more likely to get heart disease or have a stroke. The good news is that you can take steps to delay or prevent type 2 diabetes. How can I delay or prevent type 2 diabetes? You may be able to delay or prevent type 2 diabetes with: Daily physical activity, such as walking. Weight loss, if needed. Losing even a few pounds will help. Medication, if your doctor prescribes it. If you have prediabetes, these steps may bring your blood glucose to a normal range. But you are still at a higher risk for type 2 diabetes. Regular physical activity can delay or prevent diabetes. Being active is one of the best ways to delay or prevent type 2 diabetes. It can also lower your weight and blood pressure, and improve cholesterol levels. Ask your health care team about activities that are safe for you. -

Insulin Sensitivity and Resistin Levels in Gestational Diabetes Mellitus And
European Journal of Endocrinology (2008) 158 173–178 ISSN 0804-4643 CLINICAL STUDY Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition Ana Megia, Joan Vendrell, Cristina Gutierrez, Modest Sabate´1, Montse Broch, Jose´-Manuel Ferna´ndez-Real2 and Inmaculada Simo´n Endocrinology and Diabetes Research Department, University Hospital of Tarragona ‘Joan XXIII’, ‘Pere Virgili’ Institute, ‘Rovira i Virgili’ University, 43007, Tarragona, Spain, 1Laboratory Department, Hospital ‘ St Pau i Sta. Tecla’, 43003, Tarragona, Spain and 2Endocrinology and Diabetes Unit, University Hospital ‘Josep Trueta’, 17007, Girona, Spain (Correspondence should be addressed to A Megia who is now at Seccio´ d’endocrinologı´a, Hospital Universitari ‘Joan XXIII’ de Tarragona, c/Mallafre´ Guasch, 4.43007 Tarragona, Spain; Email: [email protected]) Abstract Context: Resistin is expressed and secreted by the placenta during pregnancy. Increased serum resistin levels have been found in the second half of normal pregnancy, but its role in the pathogenesis of the insulin resistance of pregnancy is undetermined. Objective: The objective of the study was to assess the relationship between circulating resistin levels and insulin sensitivity in gestational diabetes mellitus (GDM). Design and setting: A case (nZ23)–control (nZ35) study was performed at the obstetrics and endocrinology clinic of a university hospital. Patients: In total, 58 Caucasian women with a singleton pregnancy who had been referred for a 100 g oral glucose tolerance test were enrolled between the weeks 26 and 30, and 22 women with GDM were also evaluated after pregnancy. Main outcome measures: Serum resistin and insulin sensitivity in GDM during and after pregnancy. The relationship of resistin to metabolic abnormalities was evaluated. -

What Everyone Needs to Know About Prediabetes
diaTribe.org provides free cutting-edge diabetes insights and actionable tips for people with diabetes. Our mission is to help individuals better understand their diabetes and to make our readers happier & healthier. What Everyone Needs to Know about Prediabetes: Symptoms, Diagnosis, Prevention, and more! What is prediabetes? Prediabetes is a condition where blood sugar levels are higher than normal, but not high enough to be diagnosed as type 2 diabetes. This occurs when the body has problems in processing glucose properly, and sugar starts to build up in the bloodstream instead of fueling cells in muscles and tissues. Insulin is the hormone that tells cells to take up glucose, and in prediabetes, people typically initially develop insulin resistance (where the body’s cells can’t respond to insulin as well), and over time the ability to produce sufficient insulin is reduced (if no actions are taken to reverse the situation). People with prediabetes also commonly have high blood pressure as well as abnormal blood lipids (e.g. cholesterol.) These often occur prior to the rise of blood glucose levels. What are the symptoms of prediabetes? People typically do not have symptoms of prediabetes, which is partially why up to 90% of people don’t know they have it. The ADA reports that some people with prediabetes may develop symptoms of type 2 diabetes, though even many people diagnosed with type 2 diabetes show little or no symptoms initially at diagnosis. These symptoms include: Urinating often Blurry vision Feeling very thirsty Feeling very hungry, even Cuts/bruises that Tingling, pain, or though you are eating are slow to heal numbness in the hands/ feet Extreme fatigue /diaTribeNews @diaTribeNews diaTribe.org “ People typically do not have symptoms of prediabetes, which is partially why up to 90% of people don’t know they have it.” Who is at risk of developing prediabetes? Overweight and obese adults (a BMI >25 kg/m2) are at significantly greater risk for developing prediabetes, as well as people with a family history of type 2 diabetes. -

Am I at Risk for Gestational Diabetes?
Am I at risk for gestational diabetes? U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH Eunice Kennedy Shriver National Institute of Child Health and Human Development What is gestational diabetes? Gestational diabetes (pronounced jess-TAY-shun-ul die-uh-BEET-eez) is a type of high blood sugar that only pregnant women get. In fact, the word “gestational” means pregnant. If a woman gets high blood sugar when she’s pregnant, but she never had high blood sugar before, she has gestational diabetes. Between 2 percent and 10 percent of U.S. pregnancies are affected by the condition every year,1 making it one of the top health concerns related to pregnancy. If not treated, gestational diabetes can cause problems for mothers and babies, some of them serious. But there is good news. Most of the time, gestational diabetes goes away after the baby is born. The changes in your body that cause gestational diabetes normally occur only when you are pregnant. After the baby is born, your body goes back to normal and the condition goes away. Gestational diabetes is treatable, and the best outcomes result from careful management and control of blood sugar levels. The best way to control gestational diabetes is to find out you have it early and start treatment quickly. Treating gestational diabetes—even if you don’t have any symptoms or your symptoms are mild— greatly reduces health problems for mother and baby. Why do some women get gestational diabetes? Usually, the body breaks down much of the food you eat into a type of sugar, called glucose (pronounced GLOO-kos). -

1,5-Anhydroglucitol (1,5-Ag) and Its Usefulness in Clinical Practice
Medical and Biological Sciences, 2012, 26/3, 11-17 REVIEW / PRACA POGLĄDOWA Anna Maria Dąbrowska, Jerzy S. Tarach, Maria Kurowska 1,5-ANHYDROGLUCITOL (1,5-AG) AND ITS USEFULNESS IN CLINICAL PRACTICE 1,5-ANHYDROGLUCITOL (1,5-AG) I JEGO ZASTOSOWANIE W PRAKTYCE KLINICZNEJ Department of Endocrinology, Medical University, Lublin, Poland Head: Prof. dr hab. med. Andrzej Nowakowski Summary 1,5-anhydroglucitol (1,5-AG) is a major polyol in human In clinical practice, 1,5-AG is a good marker of short-term serum, structurally similar to D-glucose. The 1,5-AG pool can episodes of hyperglycaemia such as postprandial be determined by the balance between oral intake and urinary hyperglycaemia and other short term glycaemia excursions, excretion. It is derived mainly from dietary sources with a for example during pregnancy or in pre-conception, before half-life of about 1-2 weeks. 1,5-AG is not metabolized, operations and after glycaemia-related therapeutic changes. It whereas it is excreted in urine and nearly 99.9% of it is has been also reported as a marker of first-ever cardiovascular reabsorbed by the kidneys. When serum glucose level exceeds disease, a clinical test to differentiate subtypes of diabetes and the renal threshold for glucosuria, urinary glucose suppresses to monitor type 1 diabetes in children. Despite small tubular reabsorbing of 1,5-AG, leading to a loss of it in the limitations such as end-stage renal disease or liver cirrhosis, urine and a rapid reduction of this polyol in serum levels. which should be taken into consideration when interpreting Therefore, the plasma 1,5-AG concentration indirectly reflects levels of 1,5-AG, it seems to be a useful and sensitive marker episodes of hyperglycaemia. -

Insulin Resistance Syndrome GOUTHAM RAO, M.D., University of Pittsburgh Medical Center–St
CLINICAL OPINION Insulin Resistance Syndrome GOUTHAM RAO, M.D., University of Pittsburgh Medical Center–St. Margaret, Pittsburgh, Pennsylvania Insulin resistance can be linked to diabetes, hypertension, dyslipidemia, cardiovascular disease and other abnormalities. These abnormalities constitute the insulin resistance OA patient infor- syndrome. Because resistance usually develops long before these diseases appear, mation handout on insulin resistance syn- identifying and treating insulin-resistant patients has potentially great preventive drome, written by the value. Insulin resistance should be suspected in patients with a history of diabetes in author of this article, first-degree relatives; patients with a personal history of gestational diabetes, poly- is provided on page cystic ovary syndrome or impaired glucose tolerance; and obese patients, particularly 1165. those with abdominal obesity. Present treatment consists of sensible lifestyle changes, including weight loss to attain healthy body weight, 30 minutes of accumulated mod- erate-intensity physical activity per day and increased dietary fiber intake. Pharma- cotherapy is not currently recommended for patients with isolated insulin resistance. (Am Fam Physician 2001;63:1159-63,1165-6.) besity, type 2 diabetes melli- malities, including obesity, hypertension, tus (formerly known as dyslipidemia and type 2 diabetes, that are non–insulin-dependent dia- associated with insulin resistance and com- betes), hypertension, lipid pensatory hyperinsulinemia. However, a disorders and heart disease cause-and-effect relationship between in- Oare common in most Western societies and are sulin resistance, these diseases and the mech- collectively responsible for an enormous bur- anisms through which insulin resistance den of suffering. Many people have more than influences their development has yet to be one—and sometimes all—of these condi- conclusively demonstrated. -

Diabetes Management: Directory of Provider Resources (PDF)
DIABETES MANAGEMENT: DIRECTORY OF PROVIDER RESOURCES 2 Diabetes Management: Directory of Provider Resources ACKNOWLEDGMENTS Diabetes Management: Directory of Provider Resources was prepared with input from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; Centers for Medicare & Medicaid Services Advisory Panel on Outreach and Education; NORC at the University of Chicago; Jamie Murkey, MPH, PhD, Program Alignment and Partner Engagement Group 2019 summer intern; Asian Services in Action – International Community Health Center; Chinatown Public Health Center; Colorado Coalition for the Homeless; Garden City Community Health Center, Genesis Family Health; Jackson Medical Mall and Jackson Hinds Comprehensive Health Center; Montefiore Medical Center; Nash Health Care Systems; Sun Life Family Health Center; and Bryan W. Whitfield Memorial Hospital, Tombigbee Healthcare Authority. 3 Diabetes Management: Directory of Provider Resources PURPOSE The purpose of this directory is to support providers and care teams by identifying resources on the management of type 2 diabetes. It is particularly suited for providers who work with Medicare beneficiaries and vulnerable populations for whom the prevalence of type 2 diabetes and diabetes complications is higher. This directory will help the care team identify resources to improve diabetes management by promoting medication adherence. This directory also aims to equip primary care teams with tools to manage diabetes and that patients with more complex needs are appropriately referred to specialists. While some patients require care from endocrinologists, primary care teams can effectively manage many patients with prediabetes and type 2 diabetes.i Other health professionals and patients can play an important role in facilitating medication management and other diabetes self-care behaviors. -

Dietary Modulation of Insulin and Glucose in Prediabetes
24 Review Article Volume 25, Number 1, 2010 JOM Dietary Modulation of Insulin and Glucose in Prediabetes Author: Jack Challem Post O!ce Box 30246, Tucson AZ 85751 USA www.jackchallem.com Abstract Prediabetes is usually intertwined with overweight, and both conditions presage type 2 diabetes, coronary artery disease, and many other common diseases. Early signs of prediabetes include abdominal adiposity, mood swings, sugar and carbohydrate cravings, and feeling tired or mentally fuzzy after eating. Standard measures of glucose intolerance, such as fasting glucose, may reveal a “false normal” when a person is actually prediabetic. Combined with tests for glucose, fasting insulin becomes a powerful predictor of type 2 diabetes and sequelae 10 to 15 years before glucose becomes elevated. Such an early warning provides a window to implement dietary changes to restore nor- mal glucose tolerance. Adopting a Paleolithic-style diet that emphasizes fresh, low-fat animal pro- teins and high-fiber vegetables can usually reverse prediabetes. Furthermore, dietary supplements can enhance insulin sensitivity and/or glucose control. !ese supplements include alpha-lipoic acid, chromium, biotin, silymarin, vitamin D3 combined with calcium, and vitamin K. Prediabetes is an opportunity to improve health, and it is wise to seize this opportunity. Not doing so will eventually lead to type 2 diabetes, which is far more difficult to reverse and almost always requires some form of pharmaceutical intervention. Introduction year, one million Americans graduate from Prediabetes, not influenza, is the true prediabetes to type 2 diabetes. Similar trends pandemic. It is intertwined with overweight, are occurring in Canada.1 and both conditions presage type 2 diabetes Depending on the diagnostic test used, mellitus and many other health problems.