The Distribution of Cultivated and Uncultivated Cyanobacteria And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Impacts on Geyser Basins
volume 17 • number 1 • 2009 Human Impacts on Geyser Basins The “Crystal” Salamanders of Yellowstone Presence of White-tailed Jackrabbits Nature Notes: Wolves and Tigers Geyser Basins with no Documented Impacts Valley of Geysers, Umnak (Russia) Island Geyser Basins Impacted by Energy Development Geyser Basins Impacted by Tourism Iceland Iceland Beowawe, ~61 ~27 Nevada ~30 0 Yellowstone ~220 Steamboat Springs, Nevada ~21 0 ~55 El Tatio, Chile North Island, New Zealand North Island, New Zealand Geysers existing in 1950 Geyser basins with documented negative effects of tourism Geysers remaining after geothermal energy development Impacts to geyser basins from human activities. At least half of the major geyser basins of the world have been altered by geothermal energy development or tourism. Courtesy of Steingisser, 2008. Yellowstone in a Global Context N THIS ISSUE of Yellowstone Science, Alethea Steingis- claimed they had been extirpated from the park. As they have ser and Andrew Marcus in “Human Impacts on Geyser since the park’s establishment, jackrabbits continue to persist IBasins” document the global distribution of geysers, their in the park in a small range characterized by arid, lower eleva- destruction at the hands of humans, and the tremendous tion sagebrush-grassland habitats. With so many species in the importance of Yellowstone National Park in preserving these world on the edge of survival, the confirmation of the jackrab- rare and ephemeral features. We hope this article will promote bit’s persistence is welcome. further documentation, research, and protection efforts for The Nature Note continues to consider Yellowstone with geyser basins around the world. Documentation of their exis- a broader perspective. -

Yellowstone National Park Is in the U.S
english for everyone.org Name________________ Date________________ “Yellowstone”e Reading Comprehension – Informational Passages (5) Directions: Read the passage. Then answer questions about the passage below. Yellowstone National Park is in the U.S. states of Wyoming, Idaho, and Montana. It became the first National Park in 1872. There are geysers and hot springs at Yellowstone. There are also many animals at Yellowstone. There are elk, bison, sheep, grizzly bears, black bears, moose, coyotes, and more. More than 3 million people visit Yellowstone National Park each year. During the winter, visitors can ski or go snowmobiling there. There are also snow coaches that give tours. Visitors can see steam (vapor water) come from the geysers. During other seasons, visitors can go boating or fishing. People can ride horses there. There are nature trails and tours. Most visitors want to see Old Faithful, a very predictable geyser at Yellowstone. Visitors can check a schedule to see the exact time that Old Faithful is going to erupt. There are many other geysers and boiling springs in the area. Great Fountain Geyser erupts every 11 hours. Excelsior Geyser produces 4,000 gallons of boiling water each minute! Boiling water is 100 degrees Celsius, or 212 degrees Fahrenheit – that’s very hot! People also like to see the Grand Prismatic Spring. It is the largest hot spring in the park. It has many beautiful colors. The beautiful colors are caused by bacteria in the water. These are forms of life that have only one cell. Different bacteria live in different water temperatures. Visiting Yellowstone National Park can be a week–long vacation or more. -

PART 1A. 1949-1961 CHAPTER 1. Overcome with Yellowstone Fever
1 PART 1A. 1949-1961 CHAPTER 1. Overcome with Yellowstone Fever; Preparation for a Career (1949-53) During my childhood and school years prior to college, I lived with my parents, George and Katherine, and my sister, Mary Joan (3 yrs younger), in Denver, Colorado. As a young boy, I had been to Rocky Mountain National Park (RMNP)(only a few hours drive from Denver) with my parents on a number of occasions. The area’s significance as a national park did not seem to be part of my parents' awareness, at least it never was expressed to me. My parents and grandparents all referred to such trips as going to "Estes," because the town of Estes Park was on the eastern border of the Park. Occasionally we went to Grand Lake, on the western border of the Park, where some relatives resided. My parents were largely urban-oriented people. Both had graduated from South High School in Denver, but the Depression precluded either from a university education. Immediately upon high school graduation (1929) my Dad went to work for the International Trust Company (a Denver bank) as a message runner. His father, Henry, was a teller at the same bank. My Dad's grandfather George Sr. had been a bank manager and the mayor in Idaho Springs, Colorado, where my Dad was born. Dad remained a bank employee until he retired in 1981. By that time, he was very upset by banking’s expansion into the stock market and other fields. He believed this was at the expense of traditional services to customers. -

Life at High Temperatures Biotechnology in Yellowstone
Life at High Temperatures by Thomas D. Brock Biotechnology in Yellowstone © 1994 Yellowstone Association for Natural Science, History & Education, Inc. Yellowstone National Park, Wyoming 82190. The thermophilic bacteria that live in the Yellowstone hot springs have been the foundation of impressive developments in medicine and biotechnology. The unique thermostable enzymes of these bacteria are finding wide industrial and medical use, and have become the basis of a multimillion dollar industry! When researchers began to study the biology of Yellowstone hot springs in the 1960s, the presence of these hyperthermophilic bacteria was not suspected. The upper temperature limit for life was thought to be around 73 degrees C (163 degrees F), which was actually the limit for photosynthetic organisms such as cyanobacteria. The preferred temperature for thermophilic bacteria was considered even lower, around 55 degrees C (131 degrees F). Because of the known effects of heat on biological structures such as proteins and DNA, it was thought that life at higher temperatures would be impossible. In fact, biochemists have known for over 100 years that enzymes (key cellular proteins) are destroyed by boiling. However, field observations in Yellowstone showed that in certain springs bacteria existed at much higher temperatures. Although these springs were rather small and not especially conspicuous among the impressive geysers and giant hot pools of the Yellowstone thermal basins, they were impressive microbial culture systems and turned out to be of great scientific and intellectual interest. It turns out that the enzymes of Yellowstone thermophiles are very tolerant of heat and are active even at boiling water temperatures. -

T:J~ 455 HI~: ~ Ll Lows+One in STORAGE
YELL T:J~ 455 HI~: ~ ll lows+one IN STORAGE HOT SPRING ACTIVITY IN THE GEYSER BASINS OF THE FIREHOLE RIVER FOR THE 1960 SEASON .GEORGE D. MARLER l i.J\SE RETURN TO: TECHNICAL INFOm.iATION CENTER oru MIC. '"'?~ .. J DENVER SERVICE CEmER NATIONAL PARK• SERVICE Hot Spring Activity In The Geyser Basins Of The Firehole River For The 1960 Season George D. Marler* The hot springs in the geyser basins of the Firehole River continued to show marked effects and alterations due to the Hebgen Lake earthquake of the previous year. In general, the springs which did not return to near pre-quake status within a few days to about 6 weeks following the quake continued during all of 1960 to persist, with modifications, in the changes that had been induced in them. Many alterations in hot spring activity that resulted from the earth quake were latent in character. Days, weeks and sometimes months passed before these changes became evident. Some of the changes in hydrothermal functioning during 1960 must be ascribed to alterations in ground structure produced by the earthquake. These changes resulted in new foci of expression of the thermal energy. In many places deep seated fracturing has so altered former avenues of steam egress that' there is little or no liklihood that conditions in the geyser basins will ever be the same as before the big tremor. There is~ high degree of propability that it will be several years befor~ the hot springs along the Firehole River become what might be called stabilized from the effects of the 1959 ear~hquake. -

Yellowstone National Park Geology Records Preliminary Inventory Hailey Galper June 2016 Box 1
Yellowstone National Park Geology Records Preliminary Inventory Hailey Galper June 2016 Box 1: John Good. Geology field notes. Photographs, notes, correspondence – Grasshopper Glacier, field notes, photographs, An Interpreter’s Guide to Yellowstone National Park partial draft - Yellowstone, maps and photographs – Sunlight Glacier. Paper records, photographs, maps. 1961-1967 Box 2: Norris Geyser Basin Observation Logs. Paper records – notebooks. 1964-1969 Box 3: Mike Keller. Observation notes and reports, reports on thermal cleaning and possible projects for 2000. Mary Ann Moss. Report on Giant Geyser eruption in November 1996. Tim Thompson. Reports on Ferris Pool and surrounding thermal area. Rocco Paperiello. Observation notes, Giant and Giantess geysers. Records pertaining to Fairyland Basin. Paper records. 1997-2000 Box 4: Geyser observation cards. Arranged alphabetically by geyser name. Handwritten and typed. Paper records. 1935-1937 Box 5: Norris Geyser Basin observation logs. Norris Geyser Basin Museum log books. Paper records – notebooks. 1990-2009 Box 6: Geology reports 2002-2013. **Separation sheet – Geology field reports 2013-2015, Rick Hutchinson notes, 2015. PDF Electronic only. Can be found at: O:/Archives/Digital_Copies/RG1Geo/Geology Reports2013_2015 then by year and title. Paper records and PDF. 2002-2015 Box 7: Mary Ann Moss. Giant Geyser observation records (1989). Norris Geyser Basin thermal feature graphs (2001) and Thermal Reports (1986-1987). George D. Marler. Reports, “Norris Geyser Basin”, “Hot Spring Activity in the Geyser Basins of the Firehole River for the 1960 Season”, “Are Yellowstone Geysers Declining in Activity? (1947)”, presentation notes. Publications. “The Hot Springs of the Yellowstone National Park.” – Carnegie Institution of Washington News Service Bulletin 1936. -

YELLOWSTONE NATIONAL PARK R N C Hard-Sided Camping Units Only C E Perc an Ez Pelic N See Detail Map Above PE L LICAN
Services and Facilities Mammoth Hot Springs Old Faithful Canyon Village West Thumb Fishing Bridge, Lake Village 6239ft 1902m 7365ft 2254m 7734ft 2357m and Grant Village 7733ft 2357m and Bridge Bay 7784ft 2373m 0 0.1 0.5 Km To Gardiner To Madison To Tower-Roosevelt To Lake Village 0 0.5 Km To Canyon Emergencies Check the park news- Mammoth Hot Springs Hotel Grand Geyser Amphitheater For medical or other emer- paper for seasonal dates Fi Duck 0 0.1 Mi 0.5 Post Amphitheater re To Norris 0 0.5 Mi h gencies contact a ranger or of services and facilities. Office o Lake Fishing Bridge le West Thumb Ice Visitor Education call 307-344-7381 or 911. Geyser Amphitheater Hill West Thumb Castle Center Showers-Laundry Ranger station To Geyser Basin Geyser Ri Old Ice Upper Terrace Drive: Park ve Canyon Lodge Medical clinic r Faithful Visitor Headquarters no buses, RVs, or trailers; y Information Station Campground a closed in winter Center To East HISTORIC w Bookstore - Showers Entrance Winter road closures FORT e n Laundry YELLOWSTONE o YELLOWSTONE From early November to Lodging Lake Village Post early May most park roads LOWER Office Lake Lodge UPPER TERRACES Chapel Old Faithful Inn Old Faithful LAKE are closed. The exception Food service TERRACES AREA Geyser Lower Falls is the road in the park AREA Old 308ft Upper Lookout between the North one-way Faithful Falls 94m Fishing Bridge Picnic area To Point Amphitheater North Lodge View Grand Recreational Vehicle Park Entrance and Cooke City. Inspiration hard-sided camping units only Entrance Post Office View Point It is open all year. -

Geological Survey
DEPARTMENT OF THE INTERIOR OF THE UNITED STATES GEOLOGICAL SURVEY VOL. VIII WASHINGTON GOVERNMENT FEINTING OFFICE 1889 CONTENTS. BULLETIN No. 47. 47. Analyses of Waters of the Yellowstone National Park, with an Account of the Methods of Analysis employed, by Frank Austin Gooch and James Edward Whitfield. BULLETIN No. 48. 48. On the Form and Position of the Sea Level, by Robert Simpson Woodward. BULLETIN No. 49. 49. Latitudes and Longitudes of Certain Points in Missouri, Kansas, and New Mexico, by Robert Simpson Woodward. ' BULLETIN No. 50. 50. Formulas and Tables to facilitate the Construction and Use of Maps, by Rob- > ert Simpson Woodward. BULLETIN No. 51. 51. On Invertebrate Fossils from the Pacific Coast, by Charles Abiathar White. BULLETIN No. 52. 52. Subaerial Decay of Rocks and Origin of the Red Color of Certain Formations, by Israel Cook Russell. BULLETIN No. 53. 53. The Geology of Nantucket, by Nathaniel Southgate Shaler. BULLETIN No. 54. 54. A Report on the Thermo-Electrical Measurement of High Temperatures, by Carl Barus. ' m 184340 ILLUSTRATIONS. BULLETIN No. 47. Page. FIG. 1. Apparatus for the estimation of carbonic acid........................ 16 2. Apparatus.for the estimation of boric acid............................ 18 BULLETIN No. 51. PLATE I. Ostrea Dilleri.................................................... 74 II. Ostrea Dilleri.................................................. 76 III. Fulguraria Gabbi, Fulgur Hilgardi, Trophon Condoni, Cancel- laria Turneri, Gyrodes Dowelli, Rimella macilenta, Ceratia nexilia ...................................................... 78 IV. Scobinella Dilleri, Cominella LeContei, Mesalia obsuta, Trochus (Anadema) gemiferas, Stomatia obstricta, Fauntis marcidulus, Lysis oppansus, Actaeon inornatus, Vasculum obliquum, Zir- phsea plana.................................................. 80 V. Ammonites Turneri............................................ 82 VI. Anatina sulcatina ?, Crassatella tuscana, Clisocolus dubius, C. -

Geysers-1929.Pdf
UNITED STATES DEPARTMENT OF THE INTERIOR RAY LYMAN WILBUR. SECRETARY NATIONAL PARK SERVICE HORACE M. ALBRIGHT, DIRECTOR GEYSERS OF THE YELLOWSTONE NATIONAL PARK UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON: 1929 THE NATIONAL PARKS AT A GLANCE. [Number, ai; total area, 12,113 square miles.] Area in National parks in Location. square Distinctive characteristics. order of creation. miles. Hot Springs Middle Arkansas I,Va 46 hot springs said to possess healing properties— 1832 Many hotels and boarding houses—19 bath houses under Government supervision. Yellowstone Northwestern Wyo 3-426 More geysers than in all rest of "world together— 1872 ming. Boiling springs—Mud volcanoes—Petrified f orests—Grand Canyon of the Yellowstone, re markable for gorgeous coloring—Large lakes- Many large streams and waterfalls—Vast wil derness, one of the greatest wild bird and animal preserves in world—Exceptional trout fishing. Sequoia Middle eastern Cali 604 The Big Tree National Park—Scores of sequoia 1S90 fornia. trees 20 to 30 feet in diameter, thousands over 10 feet in diameter—The General .Sherman Tree is 37.3 feet in diameter and 273.9 feet high— Towering mountain ranges including Mount Whitney, the highest peak in continental United .States — Startling precipices — Deep canyons. Yosemite Middle eastern Cali I, 126 Valley of world-famed beauty—Lofty cliffs— 1890 fornia. Romantic vistas—Many waterfalls of extraor dinary height—3 groves of big trees—High Sierra—Waterwheel falls—Good trout fishing. General Grant.. Middle eastern Cali 4 Created to preserve the celebrated General Grant 1890 fornia. Tree, maximum 40.3 feet in diameter—6 miles from Sequoia National Park. -

Grids -17Etxa4
Yellowstone NATIONAL PARK with photos by: MICHAEL MELFORD TOM MURPHY ROBBIE GEORGE SERGIO LANZA CASADO TERRY DONNELLY MICHAEL S. LEWIS RAYMOND GEHMAN GRAND PRISMATIC SPRING PHOTO BY SERGIO LANZA CASADO “The center of Yellowstone’s Grand Prismatic Spring steams at 199° Fahrenheit (93° Cel- sius), too hot for the multicol- YELLOWSTONE: ored bacteria clustering on Location: Wyoming, Idaho, and Montana the cooler perimeter. But dead Established: March 1, 1872 center is no dead zone: Billions Size: 2,221,766 acres of organisms called thermo- philes flourish in the scalding is a water.” Yellowstonegeo - logical smoking gun that illustrates how violent the Earth can be. One event overshadows all others: Some 640,000 years ago, an area many miles square at what is now the center of the park suddenly exploded. In minutes the landscape was devastated. Fast-mov- ing ash flows covered thousands of square miles. At the center only a smol- dering caldera remained, a collapsed crater 45 by 30 miles. At least two other cataclysmic events preceded this one. Boiling hot springs, fumaroles, mud spots, and geysers serve as reminders that another could occur. OLD FAITHFUL PHOTO BY TOM MURPHY “Vapor rises from Old Faith- ful, one of Yellowstone’s most popular attractions. Not the YELLOWSTONE: largest or the most regular of Location: Wyoming, Idaho, and Montana the park’s geysers, Old Faithful Established: March 1, 1872 erupts more frequently, with Size: 2,221,766 acres each blast expelling between 3,700 to 8,400 gallons (14,000 to 32,000 liters) of boiling , how- water.” Yellowstoneever, is much more than hot ground and gushing steam. -
Grand Teton National Park Area Travel Map
GRAND TETON NATIONAL PARK AREA TRAVEL MAP FISH THE FLY GUIDE Yellowstone National Park Yellowstone South Entrance SERVICE & TRAVEL G JOIN ONE OF OUR good-natured ras sy guides on a guided fishing trip WINEGAR HOLE L a Headwaters Lodge k e to one of the region’s best rivers R Flagg Ranch WILDERNESS o or remote backcountry creeks or a d lakes. Combine the serenity of the river with the thrill of the take for a vacation memory you will not John D. soon forget! We package all of your fishing CARIBOU-TARGHEE FOR INFORMATION ON Rockefeller, Jr. 8 mi/3km WEATHER AND ROAD needs to get you more time on the water. NATIONAL FOREST Memorial Parkway CONDITIONS, VISIT: fishthefly.com | 307-690-1139 jacksonholetraveler.com/map 89 Red Lodge Big MT SIGNAL MOUNTAIN 287 Sky Gardiner 212 Cooke City 191 LODGE 287 SIGNAL MOUNTAIN West LODGE is a full- Yellowstone service resort located on the shores of 14 Jackson Lake in the heart of Grand Teton Island Park 20 16 16 mi/26 km Cody Lizard National Park. Offering private showers Yellowstone and a laundromat for park travelers. 20 120 Creek ID National Park GO TO JACKSONHOLETRAVELER.COM signalmountainlodge.com J. D. Rockefeller, Jr. FOR LOCAL SPECIALS & INSIDER COVERAGE. 32 307-543-2831 Rexburg Memorial Parkway Grand Driggs 287 15 33 JACKSON Victor WY 26 26 Dubois TETON BARKER-EWING 22 Idaho Jackson LAKE FLOAT TRIPS Barker- Falls Swan Grand Teton Teton Leeks WILDERNESS “Ten of THE MOST Ewing Valley 26 Shoshone Marina National Park d a BEAUTIFUL miles of river o Alpine R Two ek in the world,” and that’s re Ocean C m Lake why we run our float National ri lg JEDEDIAH SMITH Colter Bay Pi trips only inside Grand WILDERNESS Visitor Center Teton National Park. -
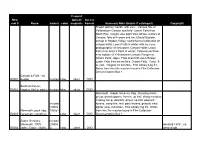
New Reel # Name Date(S)
if sound: New optical/ Access reel # Name date(s) color magnetic Format Summary Note (details if cataloged) Copyright Fragile splices; handle with care. Various film of Yellowstone Canyon and falls: Lower Falls from North Rim; canyon view point from above; visitors at Canyon; falls with snow and ice; Glacial Boulder; sunset in Hayden Valley; hydrothermal coloration on canyon walls; Lower Falls in winter with ice cone; photographer at view point; Canyon Hotel; Lower Falls from Artist's Point in winter; Yellowstone River near bottom of Yellowstone Canyon; Ranger at Artist's Point; Upper Falls and Chittenden Bridge; Lower Falls from below falls; Crystal Falls. Color, 9- in. reel. Original 16 mm film. Film Library Log # 1. Notes from this film may be found in Film Collection Documentation Box 1. Canyon & Falls - no R0001 people no date color silent DVD South Entrance, R0002 Hayden Valley, winter no date b&w silent DVD Mammoth: chapel, terraces, flag; Skinning bison; group; grasshoppers; horses: up trail, along meadow; making camp; downhill; group; up trail; pageant; no date horses; camp fire; tent; pack horses; general view; (1940s - lighter view; mountain. Film Library log #4. Notes Mammoth, pack trips, 1950s? from this film may be found in Film Collection R0003 horseman, campfires ) color silent DVD Documentation Box 1. Easter Services, no date Mammoth, 100ft - (1940s donated 1976?; no R0004 Joffe - Color - 1940s ?) color silent DVD deed of gift if sound: New optical/ Access reel # Name date(s) color magnetic Format Summary Note (details if cataloged) Copyright Approaching; camp scene; Langford; troops; horses; tents; Landford; cooks; Washburn; pitching tent; arriving; Smith; pitching tent; discussion; close up; Langford; sentry; cooks; arriving; troopers; Washburn; after discussion; Langford; retiring; Langford; cooks; troops; discussion; packers; discussion; packers; Langford; guard; Washburn.