Technical Project Lead Memorandum: SE Report SE0003731
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Academic Report Governor’S Scholars Program
2019 academic report governor’s scholars program alumni day july 13, 2019 1 Letter from the Executive Director 2 2019 Scholars by County 3 Focus Area Classes 3 Agribusiness & Biotechnology 4 Architectural Design 5 Astronomy 6 Biological & Environmental Issues 7 Business, Accounting, & Entrepreneurship 8 Communication & Social Theory 9 Creative Writing & Literary Studies 10 Cultural Anthropology 11 Dramatic Expression 12 Engineering 13 Film Studies 13 Forensic Science table of contents 15 Healthcare Industry 16 Historical Analysis 17 International Relations 18 Journalism & Mass Media 19 Modes of Mathematical Thinking 20 Music Theory & Performance 21 Philosophy 21 Physical Science 22 Political & Legal Issues 23 Psychology & Behavioral Studies 24 Spanish Language & Culture 25 Visual Arts 26 General Studies Classes 28 Scholar Experience Survey Results 32 Scholar In-State Data 33 Additional Information from the executive director Dear supporters and friends of the Governor’s Scholars Program, The 37th summer of the GSP represents a milestone in the trajectory of our beloved Program. It was a summer to renovate our commitment to the intellectual, academic, and personal growth of young leaders and maintain the unity of our learning community as “one program on three sites.” It was also a summer to honor the past and reconnect our alumni’s achievements with the bright future of many new generations to be served by the GSP. On June 13, more than 1,100 scholars—both past and present—all gathered together on Centre College’s campus for Alumni Day. Although Alumni Day has been a recurring event on our three campuses, for the first time this year we united the scholars in one place to meet with GSP alumni who have become leaders throughout our Commonwealth. -
FY 2020 Announcement.Pdf
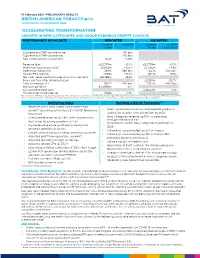
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -

Cigarette Minimum Retail Price List
MASSACHUSETTS DEPARTMENT OF REVENUE FILING ENFORCEMENT BUREAU CIGARETTE AND TOBACCO EXCISE UNIT PRESUMPTIVE MINIMUM RETAIL PRICES EFFECTIVE July 26, 2021 The prices listed below are based on cigarettes delivered by the wholesaler and do not include the 6.25 percent sales tax. Brands of cigarettes held in current inventory may be sold at the new presumptive minimum prices for those brands. Changes and additions are bolded. Non-Chain Stores Chain Stores Retail Retail Brand (Alpha) Carton Pack Carton Pack 1839 $86.64 $8.66 $85.38 $8.54 1st Class $71.49 $7.15 $70.44 $7.04 Basic $122.21 $12.22 $120.41 $12.04 Benson & Hedges $136.55 $13.66 $134.54 $13.45 Benson & Hedges Green $115.28 $11.53 $113.59 $11.36 Benson & Hedges King (princess pk) $134.75 $13.48 $132.78 $13.28 Cambridge $124.78 $12.48 $122.94 $12.29 Camel All others $116.56 $11.66 $114.85 $11.49 Camel Regular - Non Filter $141.43 $14.14 $139.35 $13.94 Camel Turkish Blends $110.14 $11.01 $108.51 $10.85 Capri $141.43 $14.14 $139.35 $13.94 Carlton $141.43 $14.14 $139.35 $13.94 Checkers $71.54 $7.15 $70.49 $7.05 Chesterfield $96.53 $9.65 $95.10 $9.51 Commander $117.28 $11.73 $115.55 $11.56 Couture $72.23 $7.22 $71.16 $7.12 Crown $70.76 $7.08 $69.73 $6.97 Dave's $107.70 $10.77 $106.11 $10.61 Doral $127.10 $12.71 $125.23 $12.52 Dunhill $141.43 $14.14 $139.35 $13.94 Eagle 20's $88.31 $8.83 $87.01 $8.70 Eclipse $137.16 $13.72 $135.15 $13.52 Edgefield $73.41 $7.34 $72.34 $7.23 English Ovals $125.44 $12.54 $123.59 $12.36 Eve $109.30 $10.93 $107.70 $10.77 Export A $120.88 $12.09 $119.10 $11.91 -

Cigarettes by Manufacturer
North Dakota Office of Attorney General Fire Marshal Division Certified Cigarettes by Manufacturer Manufacturer Brand Description Renewal British American Tobacco Singapore State Express 555 Gold Filter Box 06/21/2022 British American Tobacco Switzerland Dunhill Black Fine Cut Filter Box 06/21/2022 Dunhill Blue International Filter Box 06/21/2022 Dunhill Green International Menthol Filter Box 06/21/2022 Dunhill Red International Filter Box 06/21/2022 Dunhill White Fine Cut Filter Box 06/21/2022 Cheyenne International, LLC Aura Glen Menthol Filter Box 07/15/2022 Aura Radiant Gold Filter Box 07/15/2022 Aura Robust Red Filter Box 07/15/2022 Aura Sky Blue Filter Box 07/15/2022 Cheyenne 100's Menthol Filter Box 07/15/2022 Cheyenne Gold 100's Filter Box 07/15/2022 Cheyenne Gold Kings Filter Box 07/15/2022 Cheyenne Kings Non-Filter Box 07/15/2022 Cheyenne Kings Menthol Filter Box 07/15/2022 Cheyenne Red 100's Filter Box 07/15/2022 Cheyenne Red Kings Filter Box 07/15/2022 Cheyenne Silver 100's Filter Box 07/15/2022 Cheyenne Silver 100's Menthol Filter Box 07/15/2022 Cheyenne Silver Kings Menthol Filter Box 07/15/2022 Cheyenne Silver Kings Filter Box 07/15/2022 Decade 100's Menthol Filter Box 07/15/2022 Decade Gold 100's Filter Box 07/15/2022 Decade Gold Kings Filter Box 07/15/2022 Decade Kings Menthol Filter Box 07/15/2022 Decade Red 100's Filter Box 07/15/2022 Decade Red Kings Filter Box 07/15/2022 Decade Silver 100"s Filter Box 07/15/2022 Decade Silver 100's Menthol Filter Box 07/15/2022 Decade Silver Kings Menthol Filter Box 07/15/2022 Decade Silver Kings Filter Box 07/15/2022 Commonwealth Brands, Inc. -

Marketing of Menthol Cigarettes and Consumer Perceptions: a Review of Tobacco Industry Documents Stacey J Anderson1,2
Research paper Tob Control: first published as 10.1136/tc.2010.041939 on 19 April 2011. Downloaded from Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents Stacey J Anderson1,2 1Department of Social and ABSTRACT young, inexperienced smokers and/or Africane Behavioral Sciences, University Objective To examine tobacco industry marketing of Americans.3 In addition to youth appeal, the addi- of California, San Francisco menthol cigarettes and to determine what the tobacco tion of ‘medicinal menthol’ to cigarettes may also (UCSF), California, USA 2Center for Tobacco Control industry knew about consumer perceptions of menthol. appeal to established health-concerned smokers Research and Education, UCSF, Methods A snowball sampling design was used to who might otherwise quit.4 California, USA systematically search the Legacy Tobacco Documents Others have investigated the internal tobacco Library (LTDL) (http://legacy.library.ucsf.edu) between 28 industry documents for different but related ques- Correspondence to February and 27 April 2010. Of the approximately 11 Stacey J Anderson, Department tions on how tobacco companies manipulate of Social and Behavioral million documents available in the LTDL, the iterative menthol content in cigarettes to target young Sciences, Box 0612, University searches returned tens of thousands of results from the people5 and consumer perceptions of the sensory of California, San Francisco, CA major US tobacco companies and affiliated organisations. characteristics of menthol.6 -

Report: Changes in Nicotine Yield, 1998-2004
Changes in Nicotine Yield: 1998-2004 The Importance of Nicotine Nicotine Yield The average number of Disclosure milligrams of nicotine delivered Nicotine yield is a measure increased by 9.9% during this The Massachusetts Tobacco of the amount of nicotine in period (from 1.72 milligrams in Control Program has analyzed the smoke which a smoker 1998 to 1.89 milligrams in 2004) data from 1998-2004 and has inhales. It does not measure (t115 = 12.03, p<.0001). found that the amount of nicotine the amount of nicotine in a inhaled by the average smoker cigarette. has increased 10% over the seven Average Nicotine Yield per Cigarette year period. 1998-2004 Summary 1.95 1.9 1.89 1.85 1.84 Although per capita consumption 1.85 1.83 The amount of nicotine the 1.81 of cigarettes has declined, the smoker receives has increased 1.8 1.75 1.72 1.71 amount of nicotine consumed per Milligrams over time. Data reveal 1.7 cigarette has increased. 1.65 significant increases in nicotine 1.6 Concurrently, the amount of yield from 1998 to 2004 for all 1998 1999 2000 2001 2002 2003 2004 nicotine present in second-hand three tobacco companies smoke has also increased. (Lorillard, Philip Morris, and RJ Reynolds) and for all types of Similar increases were found for Massachusetts is one of three cigarettes -- full flavor, light, states to receive information each type of cigarette tested (full mild/medium, ultra-light; and flavor, light, mild/medium, and about nicotine levels in tobacco menthol and non-menthol. -

Page 1 of 15
Updated September14, 2021– 9:00 p.m. Date of Next Known Updates/Changes: *Please print this page for your own records* If there are any questions regarding pricing of brands or brands not listed, contact Heather Lynch at (317) 691-4826 or [email protected]. EMAIL is preferred. For a list of licensed wholesalers to purchase cigarettes and other tobacco products from - click here. For information on which brands can be legally sold in Indiana and those that are, or are about to be delisted - click here. *** PLEASE sign up for GovDelivery with your EMAIL and subscribe to “Tobacco Industry” (as well as any other topic you are interested in) Future lists will be pushed to you every time it is updated. *** https://public.govdelivery.com/accounts/INATC/subscriber/new RECENTLY Changed / Updated: 09/14/2021- Changes to LD Club and Tobaccoville 09/07/2021- Update to some ITG list prices and buydowns; Correction to Pall Mall buydown 09/02/2021- Change to Nasco SF pricing 08/30/2021- Changes to all Marlboro and some RJ pricing 08/18/2021- Change to Marlboro Temp. Buydown pricing 08/17/2021- PM List Price Increase and Temp buydown on all Marlboro 01/26/2021- PLEASE SUBSCRIBE TO GOVDELIVERY EMAIL LIST TO RECEIVE UPDATED PRICING SHEET 6/26/2020- ***RETAILER UNDER 21 TOBACCO***(EFF. JULY 1) (on last page after delisting) Minimum Minimum Date of Wholesale Wholesale Cigarette Retail Retail Brand List Manufacturer Website Price NOT Price Brand Price Per Price Per Update Delivered Delivered Carton Pack Premier Mfg. / U.S. 1839 Flare-Cured Tobacco 7/15/2021 $42.76 $4.28 $44.00 $44.21 Growers Premier Mfg. -

First-Of-Its-Kind Filing for VUSE Products
Reynolds American Inc. P.O. Box 2990 Winston-Salem, NC 27102-2990 Media Contact: Kaelan Hollon [email protected] 202-421-4921 VUSE Premarket Tobacco Product Application Filed for Substantive Review by the FDA First-of-its-Kind Filing for VUSE Products WINSTON-SALEM, N.C. – Nov. 27, 2019 – Today, Reynolds American Inc. (“RAI”) announced that the recently-submitted Premarket Tobacco Product Application (“PMTA”) for VUSE vapor products has been filed by the Food and Drug Administration (“FDA”) for substantive scientific review, moving the application one step further through the review process. “This is a first-of-its-kind application for VUSE products, and it puts VUSE one step closer to gaining a marketing order from the FDA. FDA will now review our scientific justification and determine the appropriateness of VUSE e-cigarette products against the public health standard,” notes RAI CEO Ricardo Oberlander. “For adult tobacco consumers seeking an alternative source of nicotine, having FDA oversight of e-cigarette products is an important step to ensure those alternatives meet strict regulatory scrutiny.” The FDA’s scientific review of the VUSE application – which totals more than 150,000 pages of research and data – comes just over six weeks after its initial submission to the FDA. The FDA, per its earlier-issued guidance, considers the following issues, among others: • Risks and benefits to the population as a whole, including both users and nonusers of tobacco products; • Whether people who currently use any tobacco product would be more or less likely to stop using such products if the proposed new tobacco product were available; • Whether people who currently do not use any tobacco products would be more or less likely to begin using tobacco products if the new product were available; and • The methods, facilities, and controls used to manufacture, process, and pack the new tobacco product. -

Ocm40773686.Pdf
1. Summary 2. Background 3. Nicotine Yield Testing Table I-- FTC/M Nicoline Yield Surnrnaly 4. Nicotine Content of Whole Tobacco Table 2-- Nicotine Content 5. Percent Filter Ventilation Table 3-- Filter Ventilation 6. Nicotine Yield Ratings Table 3-- Nicotine Yield Ratings 7. Appendix (a) Definitions (b) Table Summary-- Nicotine Information (c) DPH Regulations-- Testing Method for Cigarettes (d) Tobacco Industry Comments on Massachusetts Testing Nicotine Yield Testing a Massachusetts Department of Public Health developed a testing method which better simulates the smoking behavior of the average smoker under normal smoking conditions by increasing the amount of smoke inhaled with each puff by the smoking machine, reducing the amount of time taken between puffs, and requiring 50% of the cigarette filter to be covered. a Massachusetts testing for nicotine yield revealed levels about twice as high as those found by the Federal ,Trade Commission. For the average smoker, 'low yield' cigarettes deliver moderate to high doses of nicotine sufficient to cause and maintain heavy dependence. Nicotine Ran~es Since smoking behaviors vary, Massachusetts has published the range of nicotine which a cigarette delivers under average smoking conditions-- whether high, moderate, low, or nicotine ji-ee. These ranges will allow smokers to compare nicotine levels among brands of cigarettes. a 85% of the cigarettes tested in 199 7fell into the highest- nicotine ranae. Of 85 cigarette brands tested, 72 were rated as high, including many of the 'light' cigarettes tested, and even some of the 'ultra-light' cigarettes tested. 15% of cigarettes tested fell into the moderate range. Cigarettes with moderate and high yields can cause heavy dependence on nicotine. -

Section V V the DEFENDANTS CAUSED the CHARGED
Section V V THE DEFENDANTS CAUSED THE CHARGED MAILINGS AND WIRE TRANSMISSIONS IN FURTHERANCE OF THE SCHEME TO DEFRAUD A. The Charged Defendants Caused the Mailings and Wire Transmissions 1. The Court finds that, consistent with the allegations set forth in the descriptions of the Racketeering Acts below (see Section V.B), the charged Defendants performed or caused the mailings or wire transmissions described in each of those respective Racketeering Acts to be sent, delivered, or received by the requisite means of transmission consistent with 18 U.S.C. §§ 1341 and 1343. 2. Brown & Williamson has stipulated that the requirements of 18 U.S.C. § 1341 or § 1343 have been met for the following Racketeering Acts: 8, 17, 31, 32, 38, 44, 45, 50, 51, 52, 54, 57, 60, 63, 66, 67, 77, 88, 98, 103, 106, 115, 116, 118, 124, 125, 127, 129, 144. 3. BATCo has stipulated that the requirements of 18 U.S.C. § 1341 or § 1343 have been met for the following Racketeering Acts: 11, 30, 50, 51, 53, 54, 57, 60, 63, 103, and 108. 4. In their responses to Requests for Admission, various Defendants have admitted that certain of the Racketeering Acts were transmitted by the requisite means of transmission (mail or wire), including the following Racketeering Acts: 11, 26, 30, 32, 38, 44-46, 50-55, 57, 60, 63, 66-67, 70, 73, 77, 79, 81, 82, 86, 88-90, 94, 96, 98-99, 103-106, 108-110, 114, and 116. 5. For purposes of the mail fraud violations covered by 18 U.S.C. -

The African Americanization of Menthol Cigarette Use in the United States
Nicotine & Tobacco Research Volume 6, Supplement 1 (February 2004) S55–S65 The African Americanization of menthol cigarette use in the United States Phillip S. Gardiner [Received 23 December 2002; accepted 30 June 2003] Today, over 70% of African American smokers prefer menthol cigarettes, compared with 30% of White smokers. This unique social phenomenon was principally occasioned by the tobacco industry’s masterful manipulation of the burgeoning Black, urban, segregated, consumer market in the 1960s. Through the use of television and other advertising media, coupled with culturally tailored images and messages, the tobacco industry ‘‘African Americanized’’ menthol cigarettes. The tobacco industry successfully positioned mentholated products, especially Kool, as young, hip, new, and healthy. During the time that menthols were gaining a large market share in the African American community, the tobacco industry donated funds to African American organizations hoping to blunt the attack on their products. Many of the findings in this article are drawn from the tobacco industry documents disclosed following the Master Settlement Agreement in 1998. After a short review of the origins and growth of menthols, this article examines some key social factors that, when considered together, led to disproportionate use of mentholated cigarettes by African Americans compared with other Americans. Unfortunately, the long-term impact of the industry’s practice in this community may be partly responsible for the disproportionately high tobacco-related disease and mortality among African Americans generally and African American males particularly. Introduction menthol cigarettes and the adoption of these products by over 70% of African American smokers, as Mentholated cigarettes have been a ubiquitous part compared with 30% of White smokers (U.S. -

Standardised Cigarette Packaging May Reduce the Implied Safety of Natural American Spirit Cigarettes
UC San Diego UC San Diego Previously Published Works Title Standardised cigarette packaging may reduce the implied safety of Natural American Spirit cigarettes. Permalink https://escholarship.org/uc/item/16h8d1rc Journal Tobacco control, 27(e2) ISSN 0964-4563 Authors Leas, Eric Craig Pierce, John P Dimofte, Claudiu V et al. Publication Date 2018-10-01 DOI 10.1136/tobaccocontrol-2017-053940 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Downloaded from http://tobaccocontrol.bmj.com/ on January 8, 2018 - Published by group.bmj.com TC Online First, published on December 18, 2017 as 10.1136/tobaccocontrol-2017-053940 Research paper Standardised cigarette packaging may reduce the implied safety of Natural American Spirit cigarettes Eric Craig Leas,1,2,3 John P Pierce,1,2 Claudiu V Dimofte,4 Dennis R Trinidad,1,2 David R Strong1,2 1Department of Family Medicine ABSTRACT (USFDA) that they in fact reduce the health risks and Public Health, University of Background Over two-thirds of Natural American Spirit of smoking in order to be marketed as a ‘Modified California: San Diego, La Jolla, (NAS) smokers believe their cigarettes might be ’less Risk Tobacco Product’. To date, no cigarette brand California, USA 1 2UC San Diego Moores Cancer harmful’, but toxicological evidence does not support has provided such evidence. Center, University of California: this belief. We assessed whether standardised packaging In the USA, the brand ‘Natural American Spirit’ San Diego, La Jolla, California, could reduce the possibility of erroneous inferences of (NAS) is the cigarette brand most commonly USA 3 ’safety’ drawn from NAS cigarette packaging.