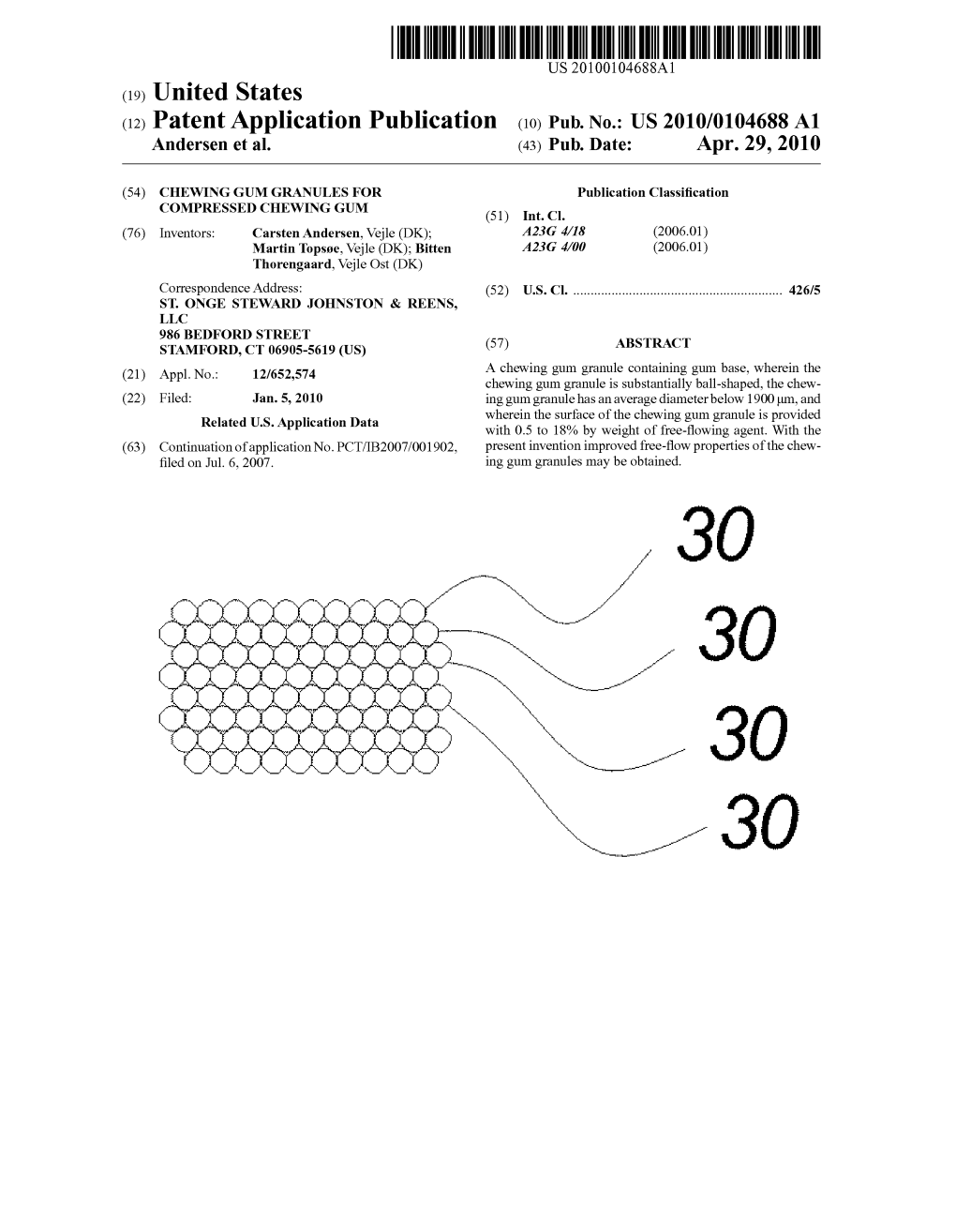
(12) Patent Application Publication (10) Pub. No.: US 2010/0104688 A1 Andersen Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Postprint : Author's Final Peer-Reviewed Version
This item is the archived peer-reviewed author-version of: Combination of miconazole and domiphen bromide is fungicidal against biofilms of resistant Candida spp. Reference: Tits Jana, Cools Freya, De Cremer Kaat, De Brucker Katrijn, Berman Judith, Verbruggen Kristof, Gevaert Bert, Cos Paul, Cammue Bruno P.A., Thevissen Karin.- Combination of miconazole and domiphen bromide is fungicidal against biofilms of resistant Candida spp. Antimicrobial agents and chemotherapy - ISSN 0066-4804 - 64:10(2020), e01296-20 Full text (Publisher's DOI): https://doi.org/10.1128/AAC.01296-20 To cite this reference: https://hdl.handle.net/10067/1705020151162165141 Institutional repository IRUA 1 Combination of miconazole and domiphen bromide is fungicidal against biofilms of resistant 2 Candida spp. 3 Jana Titsa, Freya Coolsb, Kaat De Cremera, Katrijn De Bruckera, Judith Bermanc, Kristof 4 Verbruggend, Bert Gevaertd, Paul Cosb, Bruno P.A. Cammuea, Karin Thevissena# 5 6 aCentre of Microbial and Plant Genetics, KU Leuven, Leuven, Belgium 7 bLaboratory for Microbiology, Parasitology and Hygiene (LMPH), University of Antwerp, Antwerp, 8 Belgium 9 cDepartment of Molecular Microbiology & Biotechnology, School of Molecular Cell Biology and 10 Biotechnology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel. 11 dPurna pharmaceuticals, Puurs, Belgium 12 13 Running Head: Fungicidal combination against resistant Candida spp. 14 15 #Address correspondence to Karin Thevissen, [email protected] 1 16 Abstract 17 The occurrence and recurrence of mucosal biofilm-related Candida infections, such as oral and 18 vulvovaginal candidiasis, is a serious clinical issue. Vaginal infections caused by Candida spp., for 19 example, affect 70‐75% of women at least once during their lives. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABIRATERONE 154229-19-3 ACIVICIN 42228-92-2 ABITESARTAN 137882-98-5 ACLANTATE 39633-62-0 ABLUKAST 96566-25-5 ACLARUBICIN 57576-44-0 ABUNIDAZOLE 91017-58-2 ACLATONIUM NAPADISILATE 55077-30-0 ACADESINE 2627-69-2 ACODAZOLE 79152-85-5 ACAMPROSATE 77337-76-9 ACONIAZIDE 13410-86-1 ACAPRAZINE 55485-20-6 ACOXATRINE 748-44-7 ACARBOSE 56180-94-0 ACREOZAST 123548-56-1 ACEBROCHOL 514-50-1 ACRIDOREX 47487-22-9 ACEBURIC ACID 26976-72-7 -

United States Patent to 11, 4,006,218 Sipos (45) Feb
United States Patent to 11, 4,006,218 Sipos (45) Feb. 1, 1977 54) POTENTIATED MEDICAMENTS organic halogen derivatives and iodophores derived from nonionic surface active agents and from polyvi 75) Inventor: Tibor Sipos, Jackson, N.J. nylpyrrolidone by combining the antimicrobial agent 73 Assignee: Johnson & Johnson, New Brunswick, with an effective amount of a potentiator. The potenti N.J. ator is an alcohol or phenol derivative selected from the group consisting of (a) aliphatic straight or 22 Filed: July 14, 1975 branched chain primary, secondary and tertiary mono 21 Appl. No.: 595,986 hydric alcohols wherein the straight chain alcohols have from about 5 to about 10 carbon atoms; and the branched chain alcohols have up to about 17 carbon Related U.S. Application Data atoms, their longest straight chain of carbon to carbon 63 Continuation of Ser. No. 486,287, July 8, 1974, bonds having from about 5 to about 10 carbon atoms; abandoned, which is a continuation-in-part of Ser. No. (b) a primary, secondary or tertiary cyclohexylalkanol 285,682, Sept. 1, 1972, abandoned. or alkylcyclohexylalkanol where the alkanol is as more 52 U.S. Cl. ................................. 424/54; 424/249; fully defined hereinafter; (c) a primary, secondary or 424/258; 424/263; 424/329; 424/343 tertiary phenylalkanol, halophenylalkanol or C to Ca (51 Int. Cl.’................. A61K 7/22; A61K 3 1/O45; alkylphenylalkanol where the alkanol has from about 3 A61 K. 3 1/14; AOlN 9/24 to about 9 carbon atoms; and (d) a cyclohexyl phenol 58 Field of Search ............ 424/54, 329, 343, 249, which may have a substituent on the phenyl ring se 424/258, 263 lected from the group consisting of C to Ca alkyl and alkoxy, hydroxy, halo, amino and alkyl and dialkyl (56) References Cited amino-substituents. -
Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs
Molecules 2016, 21, 75; doi:10.3390/molecules21010075 S1 of S110 Supplementary Materials: Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs Fei Mao 1, Wei Ni 1, Xiang Xu 1, Hui Wang 1, Jing Wang 1, Min Ji 1 and Jian Li * Table S1. Common names, indications, CAS Registry Numbers and molecular formulas of 6891 approved drugs. Common Name Indication CAS Number Oral Molecular Formula Abacavir Antiviral 136470-78-5 Y C14H18N6O Abafungin Antifungal 129639-79-8 C21H22N4OS Abamectin Component B1a Anthelminithic 65195-55-3 C48H72O14 Abamectin Component B1b Anthelminithic 65195-56-4 C47H70O14 Abanoquil Adrenergic 90402-40-7 C22H25N3O4 Abaperidone Antipsychotic 183849-43-6 C25H25FN2O5 Abecarnil Anxiolytic 111841-85-1 Y C24H24N2O4 Abiraterone Antineoplastic 154229-19-3 Y C24H31NO Abitesartan Antihypertensive 137882-98-5 C26H31N5O3 Ablukast Bronchodilator 96566-25-5 C28H34O8 Abunidazole Antifungal 91017-58-2 C15H19N3O4 Acadesine Cardiotonic 2627-69-2 Y C9H14N4O5 Acamprosate Alcohol Deterrant 77337-76-9 Y C5H11NO4S Acaprazine Nootropic 55485-20-6 Y C15H21Cl2N3O Acarbose Antidiabetic 56180-94-0 Y C25H43NO18 Acebrochol Steroid 514-50-1 C29H48Br2O2 Acebutolol Antihypertensive 37517-30-9 Y C18H28N2O4 Acecainide Antiarrhythmic 32795-44-1 Y C15H23N3O2 Acecarbromal Sedative 77-66-7 Y C9H15BrN2O3 Aceclidine Cholinergic 827-61-2 C9H15NO2 Aceclofenac Antiinflammatory 89796-99-6 Y C16H13Cl2NO4 Acedapsone Antibiotic 77-46-3 C16H16N2O4S Acediasulfone Sodium Antibiotic 80-03-5 C14H14N2O4S Acedoben Nootropic 556-08-1 C9H9NO3 Acefluranol Steroid -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2018 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Improved Anti-Plaque Compositions Comprising a Combination of Morpholinoamino Alcohol and Anti-Microbial Agent
Europa,schesP_ MM M II M Mill I Ml M M M M I II J European Patent Office CO Publication number: 0 510 151 B1 Office europeen„ des brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 05.04.95 © Int. CI.6: A61 K 7/22, A61 K 7/1 6 © Application number: 91919554.5 @ Date of filing: 26.09.91 © International application number: PCT/US91/07083 © International publication number: WO 92/08442 (29.05.92 92/12) IMPROVED ANTI-PLAQUE COMPOSITIONS COMPRISING A COMBINATION OF MORPHOLINOAMINO ALCOHOL AND ANTI-MICROBIAL AGENT. ® Priority: 09.11.90 US 612034 19 Fleldstone Place Flemlngton, NJ 08822 (US) @ Date of publication of application: Inventor: PAN, Pauline, H. 28.10.92 Bulletin 92/44 14 Cambridge Road Morris Plains, NJ 07950 (US) © Publication of the grant of the patent: Inventor: SHAW, Allan 05.04.95 Bulletin 95/14 24 Boxwood Drive Convent Station, NJ 07961 (US) © Designated Contracting States: Inventor: STURDIVANT, Linda, D. BE DE DK ES FR GB GR IT 268 Prospect Street East Orange, NJ 07017 (US) © References cited: EP-A- 0 373 758 US-A- 4 636 382 © Representative: Widen, Bjorn et al US-A- 4 894 221 Kabi Pharmacia AB Box 941 © Proprietor: Pharmacia AB S-251 09 Helslngborg (SE) S-171 97 Stockholm (SE) 00 @ Inventor: DILLS, Steven, S. m 18 Elmwood Drive Hackettstown, NJ 07840 (US) o Inventor: LYNCH, Donald, M. Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

WO 2019/015975 Al 24 January 2019 (24.01.2019) W ! P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2019/015975 Al 24 January 2019 (24.01.2019) W ! P O PCT (51) International Patent Classification: Kaat; Keibergstraat 2, 3360 Bierbeek (BE). COS, Paul; A61K 31/4174 (2006.01) A61K 9/107 (2006.01) Meirakkerweg 22, 2390 Oostmalle (BE). A61K 45/06 (2006.01) A61K 47/44 (2017.01) (81) Designated States (unless otherwise indicated, for every A61K 47/14 (2017.01) A61K 31/14 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 9/06 (2006.0 1) A61K 47/18 (20 17.0 1) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, PCT/EP20 18/068 192 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, (22) International Filing Date: KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, 05 July 2018 (05.07.2018) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Pharmaabkommen A1 E
Annex I - Pharmaceutical substances, which are free of duty_______________________________________________ Pharmaceutical substances which are Annex I free of duty CAS RN Name 136470-78-5 abacavir 129639-79-8 abafungin 792921-10-9 abagovomab 65195-55-3 abamectin 90402-40-7 abanoquil 183849-43-6 abaperidone 183552-38-7 abarelixe 332348-12-6 abatacept 143653-53-6 abciximab 111841-85-1 abecarnil 167362-48-3 abetimus 154229-19-3 abiraterone 137882-98-5 abitesartan 96566-25-5 ablukast 178535-93-8 abrineurin 91017-58-2 abunidazole 2627-69-2 acadesine 77337-76-9 acamprosate 55485-20-6 acaprazine 56180-94-0 acarbose 514-50-1 acebrochol 26976-72-7 aceburic acid 37517-30-9 acebutolol 32795-44-1 acecainide 77-66-7 acecarbromal 827-61-2 aceclidine 89796-99-6 aceclofenac 77-46-3 acedapsone 127-60-6 acediasulfone sodium 556-08-1 acedoben 80595-73-9 acefluranol 10072-48-7 acefurtiamine 70788-27-1 acefylline clofibrol 18428-63-2 acefylline piperazine 642-83-1 aceglatone 2490-97-3 aceglutamide 110042-95-0 acemannan 53164-05-9 acemetacin 131-48-6 aceneuramic acid 152-72-7 acenocoumarol 807-31-8 aceperone 61-00-7 acepromazine 13461-01-3 aceprometazine 42465-20-3 acequinoline 33665-90-6 acesulfame 118-57-0 acetaminosalol 97-44-9 acetarsol 59-66-5 acetazolamide 3031-48-9 acetergamine 299-89-8 acetiamine 2260-08-4 acetiromate 968-81-0 acetohexamide 546-88-3 acetohydroxamic acid 2751-68-0 acetophenazine 1 / 135 (As of: 1.4.2013) Annex I - Pharmaceutical substances, which are free of duty_______________________________________________ CAS RN Name 25333-77-1 acetorphine -

OTC Active Ingredients April 7, 2010
OTC Active Ingredients April 7, 2010 UNII Code Active Ingredient Monograph Sub-category Panel Pending Final FR Citation 18B8O9DQA2 alcloxa acne II II 310.545(a)(1) alkyl isoquinolinium bromide acne II II 310.545(a)(1) aluminum chlorohydrex acne II II 310.545(a)(1) 5QB0T2IUN0 aluminum hydroxide acne II II 310.545(a)(1) U3RSY48JW5 benzocaine acne II II 310.545(a)(1) 8SKN0B0MIM benzoic acid acne II II 310.545(a)(1) W9WZN9A0GM benzoyl peroxide acne I I-> IIIS 333.310(a) 75 FR 9767 R57ZHV85D4 boric acid acne II II 310.545(a)(1) calcium polysuldfide acne II II 310.545(a)(1) 210100728E calcium thiosulfate acne II II 310.545(a)(1) 5TJD82A1ET camphor acne II II 310.545(a)(1) BPF36H1G6S chlorhydroxyquinoline (cloxyquin) acne II II 310.545(a)(1) LJ25TI0CVT chloroxylenol acne II II 310.545(a)(1) BPF36H1G6S cloxyquin (chlorhydroxyquinoline) acne 310.545(a)(1) R533ES02EC coal tar acne II II 310.545(a)(1) Z3D4AJ1R48 dibenzothiophene acne II II 310.545(a)(1) 2DI9HA706A estrone acne II II 310.545(a)(1) 6M2P64V0NC magnesium aluminum silicate acne 310.545(a)(1) DE08037SAB magnesium sulfate acne II II 310.545(a)(1) 339NCG44TV phenol acne II II 310.545(a)(1) 4NC0T56V35 phenolate sodium acne II II 310.545(a)(1) 28A37T47QO phenyl salicylate acne II II 310.545(a)(1) 85H0HZU99M povidone-iodine acne III III 310.545(a)(1) R35D29L3ZA pyrilamine maleate acne II II 310.545(a)(1) YUL4LO94HK resorcinol (as single ingredient only) acne III II 310.545(a)(1) YUL4LO94HK resorcinol (when combined with sulfur) acne 333.310(b) YUL4LO94HK resorcinol monoacetate (as single ingredient -

)&F1y3x PHARMACEUTICAL APPENDIX to the HARMONIZED
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ADAPALENE 106685-40-9 ABANOQUIL 90402-40-7 ADAPROLOL 101479-70-3 ABECARNIL 111841-85-1 ADEMETIONINE 17176-17-9 ABLUKAST 96566-25-5 ADENOSINE PHOSPHATE 61-19-8 ABUNIDAZOLE 91017-58-2 ADIBENDAN 100510-33-6 ACADESINE 2627-69-2 ADICILLIN 525-94-0 ACAMPROSATE 77337-76-9 ADIMOLOL 78459-19-5 ACAPRAZINE 55485-20-6 ADINAZOLAM 37115-32-5 ACARBOSE 56180-94-0 ADIPHENINE 64-95-9 ACEBROCHOL 514-50-1 ADIPIODONE 606-17-7 ACEBURIC ACID 26976-72-7 ADITEREN 56066-19-4 ACEBUTOLOL 37517-30-9 ADITOPRIME 56066-63-8 ACECAINIDE 32795-44-1 ADOSOPINE 88124-26-9 ACECARBROMAL 77-66-7 ADOZELESIN 110314-48-2 ACECLIDINE 827-61-2 ADRAFINIL 63547-13-7 ACECLOFENAC 89796-99-6 ADRENALONE 99-45-6 ACEDAPSONE 77-46-3 AFALANINE 2901-75-9 ACEDIASULFONE SODIUM 127-60-6 AFLOQUALONE 56287-74-2 ACEDOBEN 556-08-1 AFUROLOL 65776-67-2 ACEFLURANOL 80595-73-9 AGANODINE 86696-87-9 ACEFURTIAMINE 10072-48-7 AKLOMIDE 3011-89-0 ACEFYLLINE CLOFIBROL 70788-27-1