Diet of the Antillean Manatee
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Red Names=Invasive Species Green Names=Native Species
CURLY-LEAF PONDWEED EURASIAN WATERMIL- FANWORT CHARA (Potamogeton crispus) FOIL (Cabomba caroliniana) (Chara spp.) This undesirable exotic, also known (Myriophyllum spicatum) This submerged exotic Chara is typically found growing in species is not common as Crisp Pondweed, bears a waxy An aggressive plant, this exotic clear, hard water. Lacking true but management tools are cuticle on its upper leaves making milfoil can grow nearly 10 feet stems and leaves, Chara is actually a limited. Very similar to them stiff and somewhat brittle. in length forming dense mats form of algae. It’s stems are hollow aquarium species. Leaves The leaves have been described as at the waters surface. Grow- with leaf-like structures in a whorled are divided into fine resembling lasagna noodles, but ing in muck, sand, or rock, it pattern. It may be found growing branches in a fan-like ap- upon close inspection a row of has become a nuisance plant with tiny, orange fruiting bodies on pearance, opposite struc- “teeth” can be seen to line the mar- in many lakes and ponds by the branches called akinetes. Thick ture, spanning 2 inches. gins. Growing in dense mats near quickly outcompeting native masses of Chara can form in some Floating leaves are small, the water’s surface, it outcompetes species. Identifying features areas. Often confused with Starry diamond shape with a native plants for sun and space very include a pattern of 4 leaves stonewort, Coontail or Milfoils, it emergent white/pinkish early in spring. By midsummer, whorled around a hollow can be identified by a gritty texture flower. -

Bioone? RESEARCH
RESEARCH BioOne? EVOLVED Proximate Nutrient Analyses of Four Species of Submerged Aquatic Vegetation Consumed by Florida Manatee (Trichechus manatus latirostris) Compared to Romaine Lettuce (Lactuca sativa var. longifolia) Author(s): Jessica L. Siegal-Willott, D.V.M., Dipl. A.C.Z.M., Kendal Harr, D.V.M., M.S., Dipl. A.C.V.P., Lee-Ann C. Hayek, Ph.D., Karen C. Scott, Ph.D., Trevor Gerlach, B.S., Paul Sirois, M.S., Mike Renter, B.S., David W. Crewz, M.S., and Richard C. Hill, M.A., Vet.M.B., Ph.D., M.R.C.V.S. Source: Journal of Zoo and Wildlife Medicine, 41(4):594-602. 2010. Published By: American Association of Zoo Veterinarians DOI: 10.1638/2009-0118.1 URL: http://www.bioone.org/doi/full/10.1638/2009-0118.1 BioOne (www.bioone.org) is an electronic aggregator of bioscience research content, and the online home to over 160 journals and books published by not-for-profit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne's Terms of Use, available at www.bioone.org/page/terms of use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Rice-Fields. the Relevance of Cyanobacteria in the Ecosystem
Limnetica 23(1-2) 11/10/04 10:15 Página 95 95 A shallow water ecosystem: rice-fields. The relevance of cyanobacteria in the ecosystem. Eduardo Fernández-Valiente* and Antonio Quesada Departamento de Biología. Universidad Autónoma de Madrid, E-28049 Madrid, Spain * Corresponding author, tel: 34-914978186, fax: 34-914978344, email: [email protected] ABSTRACT In this paper we review the knowledge of the ecology of the largest freshwater ecosystem on Earth: the rice-fields, and in particular the rice-fields from Valencia (Spain) making a special consideration to the cyanobacteria present in this ecosystem. Rice-fields are artificial shallow aquatic ecosystems in which the land management and the agricultural practices together with the rice plant growth govern the major environmental variables affecting the aquatic biota and its relationships. Primary producers are dominated typically by macrophytic algae as Chara and cyanobacteria, both planktonic and benthic (beside the rice plants). Most rice-fields can be considered nutrient replete, since the fertilization inputs and the low ratio volume/surface make that main nutrients are typically available. Under these circumstances other environmental variables as photosynthetically active radiation availability or filtration rates and predation may explain the growth limitation of primary producers. Irradiance availability identify two periods within the cultivation cycle: when plants are short, irradiance is not limiting and some water chemistry variables (as pH, oxygen and dissolved inorganic C concentrations) change drastically as a function of the primary production; when plants are large and the canopy is intense, then irradiance is limiting and the water chemistry changes only slightly along the day. -

Morphology and Biology of Some Turbellaria from the Mississippi Basin
r MORPHOLOGY AND BIOLOGY OF SOME TURBELLARIA FROM THE MISSISSIPPI BASIN WITH THREE PLATES THESIS S1IllMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1917 BY RUTH HIGLEY A. B. Grinnell College, 1911 I ContributioD3 from the Zoological Labor&toty o[ the Univenlty of Dlinois under the direction ofHC!l1Y B. Ward, No. 112 TABLE OF CONTENTS PAGE Introduction ,.......................................... 7 Technique ,.......... 9 Methods of Study ,.......... 10 Biology , '. 12 Types of Localities ,................................... 12 Reactions of Worms ,............................................... 17 Morphology , , .. ",............ 22 Family Planariidae............................... 22 Planaria velaJa Stringer 1909............................. 22 Planoria maculata Leidy 1847......................... 23 Planaria lrumata Leidy 1851........................... 24 Family Catenulidae ,,,,, 25 Stenostrmro lew;ops (Ant. Duges) 1828 .. 26 Slcnostrmro tenuuauaa von Graff 1911.. 30 Stenostrmro giganteum nov. spec .. 30 Reprinted from the Stcnostrmro glandi(erum nov. spec . 35 lllinois Biological Monographs Volume 4, number 2, pages 195-288 Family Microstomidae......... .. ... 37 without changes in text or Murostoma cauaatum Leidy 1852 . 38 illustrations Macrostrmro sensitirJUm Silliman 1884 . 39 Macrostrmro album nov. spec ... 39 Family Prorhynchidae , .. 42 Prorhynchus stagna/is M. Schultze 1851.. .. 43 Prorltymh'" applana/us Kennel 1888 , .. 44 -

Irgc News 28
IRGC NEWS INTERNATIONAL RESEARCH GROUP ON CHAROPHYTES ISSN 1834-6030 Edited by: K. Torn, S. Schneider, A. Pukacz and E. Nat 28 March 2017 CONTENTS Editorial 1 Images collection 21 New executive commitee 2 Announcement 21 In Memoriam 2 PhD thesis completion 22 Welcome to New Members 5 Forthcoming meetings 26 Minutes of the General Assembly 2016 5 Charophyte discussion forum 27 Report on past meetings 8 New IRGC homepage 28 Publication of the proceedings 7th IRGC, Astana 15 Charophytes on the web 28 Call for participation 15 Membership fees 29 Special issue: Botanica Serbica 15 E-mail addresses of IRGC members 30 Reference article 16 Address list of members 31 Report introduction 21 Group photograph 7th IRGC, Astana 36 EDITORIAL Another year has passed, and our small but very active organization has contributed with a number of exciting activities. Not at least, the 7th IRGC symposium was held in Astana, Kazakhstan. I thank the organizing committee, namely Aizhan Zhamangara, Raikhan Beisenova, Sherim Tulegenov, Leyla Akbayeva and Saida Nigmatova, for their excellent work, and the hosting institution, the L.N. Gumilyov Eurasian National University, for their hospitality. Please find the reports of the meeting in this issue of the IRGC-news; for all those who could not participate, the reports are an excellent chance to get up-to-date with the activities at the meeting, and for all others they are a wonderful op- portunity to remember the scientific exchange, the hardships of the weather, and the very nice talks with friends and colleagues. At the meeting, a new Executive Committee was elected, and I thank all voting members for the confidence placed in the new Committee. -

Chara-Nitella.Pdf
A WEED REPORT from the book Weed Control in Natural Areas in the Western United States This WEED REPORT does not constitute a formal recommendation. When using herbicides always read the label, and when in doubt consult your farm advisor or county agent. This WEED REPORT is an excerpt from the book Weed Control in Natural Areas in the Western United States and is available wholesale through the UC Weed Research & Information Center (wric.ucdavis.edu) or retail through the Western Society of Weed Science (wsweedscience.org) or the California Invasive Species Council (cal-ipc.org). Chara spp. and Nitella spp. Chara sp. Chara and nitella Family: Characeae Range: Throughout North America. Habitat: Ponds, lakes, reservoirs, rivers, streams, bogs, canals, and rice fields. Some species inhabit brackish water. Chara often grows in hard water. Nitella sp. Origin: All species are native to North America. Impacts: Chara and nitella provide food and cover for wildlife and are important components of natural aquatic ecosystems. These algae sometimes grow in rice fields and canals, but are rarely of importance as weeds. At first glance, chara and nitella are easily mistaken for vascular aquatic plants. These submerged plant-like green algae are usually anchored to the substrate by well-developed, colorless Nitella sp. rhizoids. There are several species that occur in various regions of the western United States. Central axes of chara and nitella are regularly jointed, solid between nodes, with whorls of branches at each node, to 12 inches long or more. These algae do not have leaves. Chara species are typically coarse, gray-green, sometimes encrusted with carbonates, making plants rough to touch, and often have a garlic or skunk-like odor. -

Chara Drouetii R.D
ISSN 1809-127X (online edition) © 2011 Check List and Authors Chec List Open Access | Freely available at www.checklist.org.br Journal of species lists and distribution N Charophyceae, Charales, Characeae, Chara drouetii R.D. Wood, 1965: First record from the state of Quintana Roo, ISTRIBUTIO Mexico D * RAPHIC Mitchell S. Alix and Robin W. Scribailo G EO G N Purdue University North Central, Department of Biology, 1401 South Highway 421, Westville, IN, United States 46391. O * Corresponding author. E-mail: [email protected] OTES N Abstract: Chara drouetii Roo, Mexico. This report extends R.D. Wood, the range 1965 of wasChara recently drouetii collected by approximately during floristic 300 km surveys east of ofthe aquatic nearest macrophytes known occurrence in the ofYucatán this species Peninsula, in Mexico Mexico. and This approximately discovery represents 6,000 km the northwest first documented of the type record locality for this (municipality species from ofthe Fortaleza, state of Quintana state of Ceará, Brazil). Comparative morphometric data on diagnostic taxonomic characters of this species are presented. The genus Chara Linnaeus, 1753 comprises a diverse 23 km north of Manuel, state of Tamaulipas, collected by and complex group of macrophytic green algae that Jeremy D. Pickett-Heaps (collection date absent), United occurs on every continent, excluding Antarctica, (Wood States National Herbarium, US TAMPS-14. It is one of an and Imahori 1959; Wood 1964; 1965; Mann et al. 1999; estimated 17 Chara species found in Mexico (Scribailo Martin et al. 2004). Members of this genus are found in a and Alix 2010). In this paper, we present an additional variety of natural and man-made aquatic habitats, which Chara drouetii from a wetland include fresh and brackish water systems, permanent lakes and ponds, rivers and streams, swales and ditches, fromconfirmed the state occurrence of Quintana of Roo, Mexico. -

Different Fates of the Chloroplast Tufa Gene Following Its Transfer to the Nucleus in Green Algae
Proc. Nail. Acad. Sci. USA Vol. 87, pp. 5317-5321, July 1990 Evolution Different fates of the chloroplast tufA gene following its transfer to the nucleus in green algae (elongation factor Tu/gene transfer/organelle genomes/gene duplication/multigene family) SANDRA L. BALDAUF*, JAMES R. MANHARTt, AND JEFFREY D. PALMER* *Department of Biology, Indiana University, Bloomington, IN 47405; and tDepartment of Biology, Texas A&M University, College Station, TX 77843 Communicated by David L. Dilcher, May 8, 1990 (receivedfor review March 23, 1990) ABSTRACT Previous work suggested that the tufA gene, by a combination offilter hybridization and gene sequencing. encoding protein synthesis elongation factor Tu, was trans- Among the Charophyceae, an unusual chloroplast tufAl was ferred from the chloroplast to the nucleus within the green algal found in the genus Coleochaete, the proposed sister group to lineage giving rise to land plants. In this report we investigate land plants (10, 11). the timing and mode of transfer by examining chloroplast and nuclear DNA from the three major classes of green algae, with MATERIALS AND METHODS emphasis on the class Charophyceae, the proposed sister group Filaments of Spirogyra maxima and Sirogonium melanospo- to land plants. Filter hybridizations reveal a chloroplast tufA rum were obtained from unialgal cultures grown in soil/water gene in all Ulvophyceae and Chlorophyceae and in some but not medium (12) on a 16:8 hr light: dark cycle at 200C ± 20C under all Charophyceae. One charophycean alga, Coleochaete orbic- fluorescent light at an illumination of 50 gmol m-2'so1. Ni- ularis, is shown to contain an intact but highly divergent tella translucens and Chara connivens were grown in aquaria chloroplast tufA gene, whose product is predicted to be non- in a soil/water solution in a greenhouse. -

Key to Common Species of Stonewort
KEY TO COMMON SPECIES OF STONEWORT 7 Spines sticking out from the stem, inclined more or less towards the centre of the internode, acute-tipped; (cortex even in width or with spine-bearing rows This key covers over 99% of stoneworts encountered in Britain and Ireland. Species narrower than those between) Chara hispida not included are Red Data Book or "near threatened". An asterisk indicates that a Spines appressed to stem with two of the three spines (not usually more than binocular microscope is normally required. A x20 hand lens is recommended for three) more or less pointing in opposite direction up and down the stem (in other characters. youngest, not fully expanded internodes the density of spines may push them in various directions), obtuse to acute-tipped; (spine-bearing rows much narrower 1 Main stem corticate, often spiny 2 than those between so that spines appear to be in furrows of stem) Chara rudis Main stem without cortex 8 (Non-corticate species have semi-translucent stems, like looking through a green 8 Branchlets apparently unbranched, but many with a minute tuft of 1-3 celled bottle; corticate species have more opaque stems with stripes of cells running branches at the ends, visible under a hand lens; plant robust (stem 1-3 mm down them.) diameter and internodes up to 10 cm long), translucent, usually more or less yellowish-green Nitella translucens 2 Spines and stipulodes well-developed and acute-tipped 4 Many branchlets conspicuously branched; plant slender to robust, usually grey- Spines, and usually stipulodes blunt-tipped or undeveloped 3 green, mid to dark green or black 9 (Spines are found on the main stem (cf. -
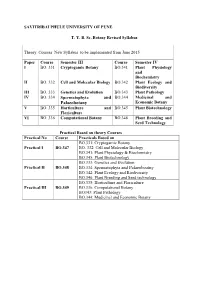
T.Y.B.Sc. Botany Revised Syllabus Semester III
SAVITRIBAI PHULE UNIVERSITY OF PUNE T. Y. B. Sc. Botany Revised Syllabus Theory Courses New Syllabus to be implemented from June 2015 Paper Course Semester III Course Semester IV I BO. 331 Cryptogamic Botany BO.341 Plant Physiology and Biochemistry II BO. 332 Cell and Molecular Biology BO.342 Plant Ecology and Biodiversity III BO. 333 Genetics and Evolution BO.343 Plant Pathology IV BO. 334 Spermatophyta and BO.344 Medicinal and Palaeobotany Economic Botany V BO. 335 Horticulture and BO.345 Plant Biotechnology Flo riculture VI BO. 336 Computational Botany BO.346 Plant Breeding and Seed Technology Practical Based on theory Courses Practical No Course Practicals Based on BO.331: Cryptogamic Botany Practical I BO.347 BO. 332: Cell and Molecular Biology BO.341: Plant Physiology & Biochemistry BO.345: Plant Biotechnology BO.333: Genetics and Evolution Practical II BO.348 BO.334: Spermatophyta and Palaeoboatny BO.342: Plant Ecology and Biodiversity BO.346: Plant Breeding and Seed tech nology BO.335: Horticulture and Floriculture Practical III BO.349 BO.336: Computational Botany BO343: Plant Pathology BO.344: Medicinal and Economic Botany Equivalence of the T.Y.B.Sc. Botany Revised Syllabus Semester III Theory Courses New Syllabus to be implemented from June 2015 Paper Course Semester III (New Course Semester III ( Old Syllabus) Syllabus) I BO. 331 Cryptogamic Botany BO. 331 Algae, Fungi and Bryophytes II BO. 332 Cell and Molecular BO. 332 Molecular Biology Biology III BO. 333 Genetics and Evolution BO. 333 Angiosperms and Evolution IV BO. 334 Spermatophyta and BO. 334 Genetics and Plant Palaeoboatny Breeding V BO. -

Sirenews (ISSN 1017-3439) Is Published in April and October and Is Edited by Cynthia R
news SNewsletterire of the IUCN Sirenia Specialist Group APRILApril 2007 2009 Funde d by the US Marine Mammal Commission NUMBER Number 51 47 IN THIS ISSUE: • WEST AFRICAN MANATEE CONSERVATION PROGRAM (pg. 3) • ABSTRACTS FROM INTERNATIONAL SIRENIAN CONFERENCE (pg. 14-29) 2009 INTERNATIONAL SIRENIAN CONSERVATION CONFERENCE ATLANTA, GEORGIA: MARCH 23-24, 2009 This meeting was the next progression from the First International Manatee and Dugong Research Conference held in Gainesville Florida, March 11-13, 1994. As the keynote speaker Tom O’Shea noted, “It has been a long 15 years since our first meeting and an abundance of new information on Sirenian natural history, anatomy, physiology, genetics, medicine and conservation is now available”. The idea for the conference was initiated by Dr. Greg Bossart and deftly organized by fellow colleagues from Harbor Branch Oceanographic Institute at Florida Atlantic University, Dr. Juli Goldstein and Stephen McCulloch. Together, they worked to acquire the necessary funding and target a select group of esteemed speakers (above) from around the world to make presentations at the renowned Georgia Aquarium. UNION INTERNATIONALE POUR LA CONSERVATION DE LA NATURE ET DE SES RESSOURCES INTERNATIONAL UNION FOR CONSERVATION OF NATURE AND NATURAL RESOURCES Commission de la sauvegarde des especes-Species Survival Commission Sirenews (ISSN 1017-3439) is published in April and October and is edited by Cynthia R. Taylor, Wildlife Trust, 233 Third St. N., Suite 300, St. Petersburg, FL 33701 USA and James A. Powell, PhD, Sea to Shore Alliance, 200 Second Ave. S., #315, St. Petersburg, FL 33701 USA Sirenews is available online at www.sirenian.org/sirenews.html Specifically, the two-day international conference combined a variety of internationally known experts to share a broad spectrum of manatee conservation issues from a global perspective.