Se of Differential Scanning Calorimetry for the Analysis of Illicit Substances and Their Adulterants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenolphthalein, 0.5% in 50% Ethanol Safety Data Sheet According to Federal Register / Vol
Phenolphthalein, 0.5% in 50% Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Issue date: 12/17/2013 Revision date: 07/21/2020 Supersedes: 01/23/2018 Version: 1.5 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Phenolphthalein, 0.5% in 50% Ethanol Product code : LC18200 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem, Inc. 1010 Jackson's Pointe Ct. Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or +1-703-741-5970 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS US classification Flammable liquids Category 3 H226 Flammable liquid and vapor Carcinogenicity Category 1A H350 May cause cancer Reproductive toxicity Category 2 H361 Suspected of damaging the unborn child. (oral) Specific target organ toxicity (single exposure) Category 1 H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Danger Hazard statements (GHS US) : H226 - Flammable liquid and vapor H350 - May cause cancer H361 - Suspected of damaging the unborn child. (oral) H370 - Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Precautionary statements (GHS US) : P201 - Obtain special instructions before use. -

The Action of the Phosphatases of Human Brain on Lipid Phosphate Esters by K
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.19.1.12 on 1 February 1956. Downloaded from J. Neurol. Neurosurg. Psychiat., 1956, 19, 12 THE ACTION OF THE PHOSPHATASES OF HUMAN BRAIN ON LIPID PHOSPHATE ESTERS BY K. P. STRICKLAND*, R. H. S. THOMPSON, and G. R. WEBSTER From the Department of Chemical Pathology, Guy's Hospital Medical School, London, Much work, using both histochemical and therefore to study the action of the phosphatases in standard biochemical techniques, has been carried human brain on the " lipid phosphate esters out on the phosphatases of peripheral nerve. It is i.e., on the various monophosphate esters that occur known that this tissue contains both alkaline in the sphingomyelins, cephalins, and lecithins. In (Landow, Kabat, and Newman, 1942) and acid addition to ox- and 3-glycerophosphate we have phosphatases (Wolf, Kabat, and Newman, 1943), therefore used phosphoryl choline, phosphoryl and the changes in the levels of these enzymes in ethanolamine, phosphoryl serine, and inositol nerves undergoing Wallerian degeneration following monophosphate as substrates for the phospho- transection have been studied by several groups of monoesterases, and have measured their rates guest. Protected by copyright. of investigators (see Hollinger, Rossiter, and Upmalis, hydrolysis by brain preparations over the pH range 1952). 4*5 to 100. Phosphatase activity in brain was first demon- Plimmer and Burch (1937) had earlier reported strated by Kay (1928), and in 1934 Edlbacher, that phosphoryl choline and phosphoryl ethanol- Goldschmidt, and Schiiippi, using ox brain, showed amine are hydrolysed by the phosphatases of bone, that both acid and alkaline phosphatases are kidney, and intestine, but thepH at which the hydro- present in this tissue. -

Student Safety Sheets Dyes, Stains & Indicators
Student safety sheets 70 Dyes, stains & indicators Substance Hazard Comment Solid dyes, stains & indicators including: DANGER: May include one or more of the following Acridine orange, Congo Red (Direct dye 28), Crystal violet statements: fatal/toxic if swallowed/in contact (methyl violet, Gentian Violet, Gram’s stain), Ethidium TOXIC HEALTH with skin/ if inhaled; causes severe skin burns & bromide, Malachite green (solvent green 1), Methyl eye damage/ serious eye damage; may cause orange, Nigrosin, Phenolphthalein, Rosaniline, Safranin allergy or asthma symptoms or breathing CORR. IRRIT. difficulties if inhaled; may cause genetic defects/ cancer/damage fertility or the unborn child; causes damages to organs/through prolonged or ENVIRONMENT repeated exposure. Solid dyes, stains & indicators including Alizarin (1,2- WARNING: May include one or more of the dihydroxyanthraquinone), Alizarin Red S, Aluminon (tri- following statements: harmful if swallowed/in ammonium aurine tricarboxylate), Aniline Blue (cotton / contact with skin/if inhaled; causes skin/serious spirit blue), Brilliant yellow, Cresol Red, DCPIP (2,6-dichl- eye irritation; may cause allergic skin reaction; orophenolindophenol, phenolindo-2,6-dichlorophenol, HEALTH suspected of causing genetic PIDCP), Direct Red 23, Disperse Yellow 7, Dithizone (di- defects/cancer/damaging fertility or the unborn phenylthiocarbazone), Eosin (Eosin Y), Eriochrome Black T child; may cause damage to organs/respiratory (Solochrome black), Fluorescein (& disodium salt), Haem- HARMFUL irritation/drowsiness or dizziness/damage to atoxylin, HHSNNA (Patton & Reeder’s indicator), Indigo, organs through prolonged or repeated exposure. Magenta (basic Fuchsin), May-Grunwald stain, Methyl- ene blue, Methyl green, Orcein, Phenol Red, Procion ENVIRON. dyes, Pyronin, Resazurin, Sudan I/II/IV dyes, Sudan black (Solvent Black 3), Thymol blue, Xylene cyanol FF Solid dyes, stains & indicators including Some dyes may contain hazardous impurities and Acid blue 40, Blue dextran, Bromocresol green, many have not been well researched. -

Phenolphthalein Is Pink in Base Acid–Base Indicators SCIENTIFIC
Phenolphthalein Is Pink in Base Acid–Base Indicators SCIENTIFIC Introduction Phenolphthalein is a large organic molecule used as an acid–base indicator. Phenolphthalein turns a bright red color as its solution becomes basic. In a strongly basic solution, this red color fades to colorless. Concepts • Acid–base indicators • Chemical equilibrium Background Phenolphthalein has the colorless structure shown in Figure 1 when the solution pH <8. As the solution becomes basic and the pH increases (pH 8–10), the phenolphthalein molecule (abbreviated H2P) loses two hydrogen ions to form the red- violet dianion (abbreviated P2–) shown in Figure 2. At a high pH, the P2– ions reacts with hydroxide ions to form the colorless POH3– ion. HO – – O –O O O OH O O– C C C – – + OH C O CO– 2 CO2 OH – CO2 O P2– Red 2– POH3– Colorless3– Figure 1. H2P is colorless. Figure 2. P is red. Figure 3. POH is colorless. 2– The colorless-to-red transition of H2P to P (Equation 1) is very rapid and the red color develops instantly when the pH reaches its transition range (pH 8–10). If the concentration of hydroxide ions remains high, the red P2– dianion will slowly combine with hydroxide ions to form a third species, POH3– (Equation 2), which is again colorless. The rate of this second reaction is much slower than the first and depends on the concentration of phenolphthalein and hydroxide ions. Thus, the color of the red P2– species will gradually fade in a basic solution. fast 2– + H2P → P + 2H Equation 1 Colorless Red slow P2– + OH– → POH3– Equation 2 Red Colorless Materials (for each demonstration) Hydrochloric acid solution, HCl, 3 M, 10 mL Beral-type pipet, disposable Phenolphthalein solution, 0.5%, 3mL Test tubes, borosilicate, 16 5 100mm, 3 Sodium hydroxide pellets, NaOH, 2 Test tube rack Sodium hydroxide solution, NaOH, 3 M, 5 mL Wash bottle Safety Precautions Hydrochloric acid solution is toxic and corrosive to eyes and skin tissue. -

Fatal Hepatitis Associated with Diclofenac
Gut: first published as 10.1136/gut.27.11.1390 on 1 November 1986. Downloaded from Gut, 1986, 27, 1390-1393 Case reports Fatal hepatitis associated with diclofenac E G BREEN, J McNICHOLL, E COSGROVE, J MCCABE, AND F M STEVENS From the Department of Medicine, Regional Hospital, Galway, Eire SUMMARY Non-steroidal anti-inflammatory agents (NSAIDS) are a well recognised cause of hepatotoxicity. Diclofenac, a relatively new NSAID, was first introduced into the UK in 1979. Five cases of hepatitis have recently been reported, principally in the French literature. -5 We report the first fulminant case of hepatitis in the English literature in a patient taking diclofenac and indomethacin. Diclofenac is a member of the arylalkanoic group of 100 mg per day for five weeks. Ferrous sulphate one NSAIDS (Fig. 1). Three other agents in this group tablet daily was added on 16 May. The patient was have been shown to be significantly hepatotoxic. admitted to hospital on 26 June. A week before this Ibufenac was withdrawn from circulation because of he had felt unwell with anorexia, nausea, abdominal the frequent rise in transaminases,6 7 the use of discomfort, and dark urine. On admission he was benoxaprofen was stopped in Britain after 10 icteric, the liver edge was palpable 4 cm below the patients died with hepatitis8 9 and more recently a costal margin and there were no signs of chronic fatal case of hepatitis due to pirprofen has been liver disease. Ultrasound showed early ascites with reported."' Early reports about diclofenac showed no obstruction of the biliary tract. -

MATERIAL SAFETY DATA SHEET Phenolphthalein
MATERIAL SAFETY DATA SHEET Phenolphthalein Section 1 Chemical Product and Company Identification MSDS Name: Phenolphthalein Catalog 147710000, 147711000, 147715000, 417180000, 417180025, 417181000, 41718 Numbers: 5000 Synonyms: Company Identification: Acros Organics BVBA Janssen Pharmaceuticalaan 3a 2440 Geel, Belgium Company Identification: (USA) Acros Organics One Reagent Lane Fair Lawn, NJ 07410 For information in the US, call: 800ACROS01 For information in Europe, call: +32 14 57 52 11 Emergency Number, Europe: +32 14 57 52 99 Emergency Number US: 2017967100 CHEMTREC Phone Number, US: 8004249300 CHEMTREC Phone Number, Europe: 7035273887 Section 2 Composition, Information on Ingredients CAS# Chemical Name: % EINECS# 77098 Phenolphthalein 2010047 Hazard Symbols: XN Risk Phrases: 22 40 Section 3 Hazards Identification EMERGENCY OVERVIEW Harmful if swallowed. Limited evidence of a carcinogenic effect. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. May be harmful if absorbed through the skin. Ingestion: Harmful if swallowed. May cause irritation of the digestive tract. Ingestion may cause fever, blood pressure increase and other unspecified vascular effects. Inhalation: May cause respiratory tract irritation. May be harmful if inhaled. Chronic: Limited evidence of a carcinogenic effect. Section 4 First Aid Measures Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: Get medical aid. Wash mouth out with water. -

And Thin-Layer Chromatography (Drug Skreen)1)
Oeilerich, Külpmann and Haeckel: Drug screening by enzyme immunoassay and .tliin-layer chromatography 275 J. Clin. Chem. Clin. Biochem. Vol. 15, 1977, pp. 275-283 Drug Screening by Enzyme Immunoassay (EMIT) and Thin-Layer Chromatography (Drug Skreen)1) By M. Oellerich, W. R. Külpmann and/?. Haeckel Technical Assistance : F. Behrends,L hberner and K. Petry Institut für Klinische Chemie (Geschäftsführcnder Direktor: Prof. Dr. Dr. J. Büttner) Medizinische Hochschule Hannover (Received June 12/December 16, 1976) Herrn Prof. Dr. Dr. h. c. G. Schettler zum 60. Geburtstag gewidmet Summary: Urine samples (n = 300) were examined for drugs by thin-layer chromatography ("Drug Skreen", Brink- mann Corp.) and by the "Enzyme Multiplied Immunoassay Technique" ("Emit", Syva Corp.). The results of both methods were compared for the detection of opiates, barbiturates and amphetamines. In more than 90% of the determinations identical results were obtained with both methods. About 10% of the results of the Emit barbiturate assay differed from those of thin-layer chromatography and therefore had to be further investigated by gas liquid chromatography. It could be demonstrated that the barbiturate determination by the Emit system correlated better with the results of gas liquid chromatography. From the results of this study it is suggested that thin-layer chromato- graphy is used as a screening test, and to confirm positive results with other methods such as Emit. If the abuse of barbiturates or opiates is suspected the corresponding Emit test should also be performed, even in cases of a negative thin-layer Chromatograph*, -screening. Confirmation with a third method such as gas liquid chromatography is necessary, if thin-layer chromatography and Emit lead to divergent results. -

The Usage of Coal-Layered Double Oxide for Removal of Toxic Dye From
Journal of Metals, Materials and Minerals, Vol. 30, No. 4, pp. 45-50, 2020 The usage of CoAl−layered double oxide for removal of toxic dye from aqueous solution Pornnapa TONGCHOO1, Sonchai INTACHAI1,*, Prakaidao PANKAM2, Chomponoot SUPPASO2, and Nithima KHAORAPAPONG2 1 Department of Chemistry, Faculty of Science, Thaksin University, Phatthalung, 93210, Thailand 2 Department of Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand *Corresponding author e-mail: [email protected] Abstract Received date: 5 July 2020 CoAl−layered double oxide derived from calcination of CoAl−layered double hydroxide at Revised date 400C for 120 min, delivered the change in microstructure with increasing surface area. Spinel Co3O4 28 October 2020 was the majority in the product as confirmed by X-ray diffraction, and Fourier transform infrared and Accepted date: UV-visible spectroscopies. The calcined product, CoAl−layered double oxide resulted in significant 31 October 2020 adsorption capacity on dye removal superior to that of CoAl−layered double hydroxide. The adsorbed amounts of the diverse dyes increased as follows: methylene blue phenolphthalein methyl orange Keywords: orange II. The adsorption affinity between adsorbent and dyes relied on electrostatic interaction Layered double oxide; and physical adsorption. Orange II; Methyl orange; Phenolphthalein; Methylene blue 1. Introduction [11-13]. The usage of LDO on the adsorptive removal of various dyes is worth investigation. Nowadays, the quality of natural water resources is numerously In this study, the adsorption of four organic dyes including degenerative. One of the possibilities may be arisen from contaminating methyl orange, orange II, methylene blue, and phenolphthalein on chemical effluents including textile dyes [1]. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -
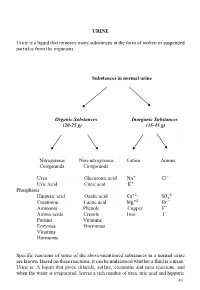
URINE Urine Is a Liquid That Removes Many Substances in the Form Of
URINE Urine is a liquid that removes many substances in the form of molten or suspended particles from the organism. Substances in normal urine Organic Substances Inorganic Substances (20-25 g) (35-45 g) Nitrogenous Non-nitrogenous Cation Anions Compounds Compounds Urea Glucuronic acid Na+ CI− Uric Acid Citric acid K+ Phosphates +2 −2 Hippuric acid Oxalic acid Ca SO4 Creatinine Lactic acid Mg+2 Br− Ammonia Phenols Copper F− Amino acids Cresols Iron I− Purines Vitamins Enzymes Hormones Vitamins Hormones Specific reactions of some of the above-mentioned substances in a normal urine are known. Based on these reactions, it can be understood whether a fluid is a urine. Urine is: A liquid that gives chloride, sulfate, creatinine and urea reactions, and when the water is evaporated, leaves a rich residue of urea, uric acid and hippuric 49 acid. Understanding whether a liquid is urine or not: •Determination of chloride, sulfate, urea, creatinine in liquid •Search for urea in the residual after the liquid has been evaporated Experiments will be carried out by sampling 2-3 ml into the tube. 1-Determination of chloride Liquid + a few drops of concentrated HNO3+ 2-3 drops of 5% AgNO3→ AgCl ↓ (white sediment) Nitric acid (HNO3 prevents the precipitation of phosphate and carbonate which could be precipitated by silver nitrate (AgNO3). 2-Determination of sulphate Liquid + several drops of 10% HCl + 2-3 drops BaCl2→ BaSO4↓ (white sediment) 3-Determination of urea Searched by two experiments: • Sodium hypobromite (NaOBr) • Using the enzyme urease a- Sodium -

Flavonols and Antioxidant Activity of Physalis Peruviana L. Fruit at Two Maturity Stages
Acta Scientiarum http://www.uem.br/acta ISSN printed: 1806-2563 ISSN on-line: 1807-8664 Doi: 10.4025/actascitechnol.v35i2.13265 Flavonols and antioxidant activity of Physalis peruviana L. fruit at two maturity stages Silvana Licodiedoff1*, Luciano André Deitos Koslowski2 and Rosemary Hoffmann Ribani1 1Programa de Pós-graduação em Tecnologia de Alimentos, Centro Politécnico, Universidade Federal do Paraná, Rua Francisco H. dos Santos, s/n, 81531-980, Cx. Postal 19011, Curitiba, Paraná, Brazil. 2Departamento de Engenharia Química, Universidade da Região de Joinville, Joinville, Santa Catarina, Brazil. *Author for correspondence. E-mail: [email protected] ABSTRACT. Since the characteristics of the fresh fruit of cape gooseberry (Physalis peruviana L.) are little known, its valorization and use are impaired. The fruit’s bioactive compounds at two stages of maturity, start and end of maturity, are evaluated, with differentiating colors between green-yellow and orange for two sizes of the fruit. The ratio between sugars and acids increased from the beginning to the end of maturity. Quercetin was not found in the samples. Nevertheless, rutin was predominant in small and large size mature sample, followed by greenish yellow (start of maturity) color of the small size fruit, with values ranging between 6.904 and 6.761 μg g-1 and 5.891 to 4.465 μg g-1, respectively. Myricetin rates ranged between 1.085 and 1.170 μg g-1 and 1.110 to 1.309 μg g-1 for greenish yellow and orange fruits, respectively. These results characterize the fruit of Physalis peruviana L. as a source of phenolic compounds in food. -

Phenolphthalein, 1% in 95% Ethanol Safety Data Sheet According to Federal Register / Vol
Phenolphthalein, 1% in 95% Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/17/2013 Revision date: 01/23/2018 Supersedes: 06/13/2014 Version: 2.1 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Phenolphthalein, 1% in 95% Ethanol Product code : LC18220 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids H225 Highly flammable liquid and vapour Category 2 Acute toxicity (oral) H302 Harmful if swallowed Category 4 Skin corrosion/irritation H315 Causes skin irritation Category 2 Serious eye damage/eye H319 Causes serious eye irritation irritation Category 2A Carcinogenicity Category 2 H351 Suspected of causing cancer (oral) Reproductive toxicity H361 Suspected of damaging fertility or the unborn child (oral) Category 2 Specific target organ H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) toxicity (single exposure) Category 1 Specific target organ H335 May cause respiratory irritation toxicity (single exposure) Category 3 Full text of H statements : see section 16 2.2.