Effect of the Upward Curvature of Toe Springs on Walking Biomechanics In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Caenagnathid Dinosaur from the Upper Cretaceous Wangshi
www.nature.com/scientificreports OPEN A new caenagnathid dinosaur from the Upper Cretaceous Wangshi Group of Shandong, China, with Received: 12 October 2017 Accepted: 7 March 2018 comments on size variation among Published: xx xx xxxx oviraptorosaurs Yilun Yu1, Kebai Wang2, Shuqing Chen2, Corwin Sullivan3,4, Shuo Wang 5,6, Peiye Wang2 & Xing Xu7 The bone-beds of the Upper Cretaceous Wangshi Group in Zhucheng, Shandong, China are rich in fossil remains of the gigantic hadrosaurid Shantungosaurus. Here we report a new oviraptorosaur, Anomalipes zhaoi gen. et sp. nov., based on a recently collected specimen comprising a partial left hindlimb from the Kugou Locality in Zhucheng. This specimen’s systematic position was assessed by three numerical cladistic analyses based on recently published theropod phylogenetic datasets, with the inclusion of several new characters. Anomalipes zhaoi difers from other known caenagnathids in having a unique combination of features: femoral head anteroposteriorly narrow and with signifcant posterior orientation; accessory trochanter low and confuent with lesser trochanter; lateral ridge present on femoral lateral surface; weak fourth trochanter present; metatarsal III with triangular proximal articular surface, prominent anterior fange near proximal end, highly asymmetrical hemicondyles, and longitudinal groove on distal articular surface; and ungual of pedal digit II with lateral collateral groove deeper and more dorsally located than medial groove. The holotype of Anomalipes zhaoi is smaller than is typical for Caenagnathidae but larger than is typical for the other major oviraptorosaurian subclade, Oviraptoridae. Size comparisons among oviraptorisaurians show that the Caenagnathidae vary much more widely in size than the Oviraptoridae. Oviraptorosauria is a clade of maniraptoran theropod dinosaurs characterized by a short, high skull, long neck and short tail. -

Percutaneous Reduction Techniques
AAOS 2016 Specialty Day Orthopaedic Trauma Associacion (OTA) Percutaneous Reduction Techniques Christian Krettek Trauma Department, Hannover Medical School, Germany [email protected] www.mhh-unfallchirurgie.de Short segment tibial fractures can be stabilized using different implants like plates, screws, external fixators and nails. This syllabus deals mainly with reduction techniques in the context of the use of intramedullary nails. 1.1 PREOPERATIVE PLANNING AND MANAGEMENT 1.1.1 Primary shortening and secondary overdistraction eases definitive nailing In the acute setting primary shortening of a shaft fracture is a helpful strategy to de- crease soft tissue tension and intra-compartmental pressure. However, reduction gets more difficult and more time consuming, the longer the fracture is kept in a shortened position. The concept of primary shortening (acute phase) and secondary (after 3 or 4 days) overdistraction eases definitive nailing. Using a electro-mechanical load cell, our group has compared the reduction forces in patients undergoing femoral nailing after Damage Control in shortend vs overdistracted fracture configuration. In the overdistrac- tion group, the reduction forces were lower (200 N +/-43.1 N vs. 336 N +/- 51.9 N, p = 0.007) and the reduction time was shorter (5.8 min +/-4.0 min vs. 28.3 min +/-21.8 min, p = 0.056). It was concluded, that DCO with the fracture shortened leads to higher restrai- ning forces & prolonged reduction time. Overdistraction should be performed as soon as possible under careful soft-tissue monitoring [1a, 2a]. Primary shortening and secondary overdistraction eases definitive nailing Example of a femoral shaft fracture stabilized in shortening first with an external fixator. -

Congenital Abnormalities of the Femur
Arch Dis Child: first published as 10.1136/adc.36.188.410 on 1 August 1961. Downloaded from CONGENITAL ABNORMALITIES OF THE FEMUR BY P. A. RING From the Royal College of Surgeons (RECEIVED FOR PUBLICATION NOVEMBER 25, 1960) Congenital defects of the femur vary from simple tion of its incidence is difficult to obtain, but it hypoplasia of the bone to complete absence. appears to be the commonest congenital defect Classification of these defects has been suggested causing major abnormalities of limb growth. by Nilsonne (1928) and by Mouchet and Ibos (1928), but neither has met with general acceptance. In more recent years Golding (1939, 1948) has demon- Clinical Features strated the close association of the short femur with There is no evidence that this is a familial disorder, congenital coxa vara, and has emphasized that and careful inquiry of the parents has revealed no these are variations of the same underlying abnor- evidence of other congenital disorders within the mality. The clinical distinction between the various immediate family. The history of the pregnancy types of femoral defect is important as a guide to and delivery has failed to indicate any significant the prognosis of limb development. infection or abnormality at this time. In most From an examination of patients with congenital patients the abnormality is apparent at birth, but abnormalities of the femur the following classifica- where the inequality of leg length is slight, the by copyright. tion is suggested: diagnosis may not be made until the child begins to 1. Simple femoral hypoplasia. walk. To ordinary clinical testing the abnormality 2. -
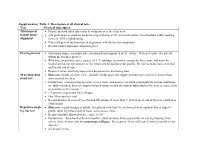
Supplementary Table 1: Description of All Clinical Tests Test Protocol
Supplementary Table 1: Description of all clinical tests Test Protocol description Tibiofemoral • Palpate & mark tibial tuberosity & midpoint over the talus neck frontal plane • Ask participant to stand on footprint map with foot at 10° external rotation, feet shoulder width, looking alignment forward, 50% weightbearing • Place callipers of inclinometer in alignment with the the two landmarks • Record varus/valgus direction in degrees Herrington test • Participant supine on plinth, knee positioned and supported in 20° of knee flexion (to place the patella within the trochlea groove) • With knee in position, place a piece of 1” Leukotape (or similar) across the knee joint, and mark the medial and lateral epicondyles of the femur and mid-point of the patella. Be sure to make note of medial and lateral end of tape • Repeat 3 times, attaching tape to this document for measuring later 30 second chair • Shoes on, middle of chair, feet ~ shoulder width apart and slightly behind knees with feet flat on floor, stand test arms crossed on chest • Instructions “stand up keeping arms across chest, and ensure you stand completely up so hips and knees are fully extended; then sit completely back down, so that the bottom fully touches the seat, as many times as possible in 30 seconds,” • 1-2 practice repetitions for technique • One 30-second test trial • Record number of correctly performed full stands (if more than ½ of way up at end of the test, counted as a full stand) Repetitive single • Shoes on, seated on edge of plinth, foot placed with heel 10 cm forward from a plumb line at edge of leg rise test plinth, other leg held at side of body, arms across chest. -

Endoscopic Repair of Full-Thickness Gluteus Medius Tears Benjamin G
Endoscopic Repair of Full-Thickness Gluteus Medius Tears Benjamin G. Domb, M.D., and Dominic S. Carreira, M.D. Abstract: Tears in the gluteus medius and minimus tendons recently have emerged as an important cause of chronic greater trochanteric pain syndrome. Increasing recognition of the gluteal insertion as a cause of chronic pain and weakness, as well as technologic advances in endoscopic hip surgery, has made gluteal insertional repair a rapidly emerging technique in minimally invasive surgery of the hip. We present an endoscopic double-row technique for gluteal insertional repair that allows for visualization, debridement, and repair, re-creating the normal footprint. ears in the gluteus medius and minimus tendons most patients respond favorably.7 A survey of French Trecently have emerged as an important cause of surgeons reporting the results of open repairs in 29 chronic greater trochanteric pain syndrome. Histori- patients showed 12 excellent results, 6 good outcomes, cally, pain over the greater trochanter was presumed and 11 poor outcomes.9 Endoscopic techniques have solely to be due to bursitis, but several studies have included gluteal debridement or repairs, bursectomy, challenged this and shown gluteus tears as a source of and iliotibial band release.10 Voos et al.11 described pain.1 Degenerative tears occur more often than acute a technique of endoscopic repair of the gluteal insertion tears,2,3 and gluteus medius tears occur more often with complete relief of symptoms in 10 patients. The than gluteus minimus tears.4,5 Tears at the insertion of advantages and limitations of endoscopic repair of the the gluteus medius can be intrasubstance, partial, or gluteus medius are described in Table 1. -
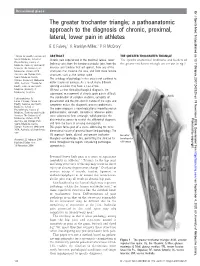
The Greater Trochanter Triangle
Occasional piece Br J Sports Med: first published as 10.1136/bjsm.2007.042325 on 19 November 2008. Downloaded from The greater trochanter triangle; a pathoanatomic approach to the diagnosis of chronic, proximal, lateral, lower pain in athletes E C Falvey,1 A Franklyn-Miller,1 P R McCrory2 1 Centre for Health, Exercise and ABSTRACT THE GREATER TROCHANTER TRIANGLE Sports Medicine, School of Chronic pain experienced in the proximal, lateral, lower The specific anatomical landmarks and borders of Physiotherapy, Faculty of Medicine, Dentistry and Health limb may arise from the femoro-acetabular joint, from the the greater trochanter triangle are set out in fig 1. Sciences The University of muscles and tendons that act upon it, from any of the Melbourne, Victoria 3010 structures that traverse the area, and from more remote Australia and Olympic Park structures such as the lumbar spine. Sports Medicine Centre, The aetiology of pathology in this area is not confined to Olympic Boulevard, Melbourne 3004, Australia; 2 Centre for either trauma or overuse. As a result many different Health, Exercise and Sports sporting activities may have a causal role. Medicine University of Without a clear clinical/pathological diagnosis, the Melbourne, Australia subsequent management of chronic groin pain is difficult. Correspondence to: The combination of complex anatomy, variability of Eanna C Falvey, Centre for presentation and the non-specific nature of the signs and Health, Exercise and Sports symptoms makes the diagnostic process problematic. Medicine, School of Physiotherapy, Faculty of The paper proposes a novel educational model based on Medicine, Dentistry and Health pathoanatomic concepts. -

Bone Handout”)
The Skeletal System (“Bone Handout”) Bone Markings - as in Table 6.1, know categories, names, descriptions and categories of bone markings Common Fractures - as in Table 6.2, identify type from pictures The Axial Skeleton Skull Cranial bones frontal (supraorbital foramen) parietal temporal (mastoid process, external auditory(acoustic) meatus, styloid process, zygomatic process, stylomastoid foramen) occipital (foramen magnum, hypoglossal canal, occipital condyles) sphenoid (optic canal, superior orbital fissure, sella turcica, foramen rotundum, foramen ovale, foramen spinosum , greater wings, lesser wings) ethmoid (olfactory foramina) Relevant Figures: 7.4, 7.6, 7.7a, 7.9 Facial bones nasal maxilla (infraorbital foramen, inferior orbital fissure) zygomatic mandible (mental foramen, body, ramus, mandibular condyle) lacrimal (lacrimal fossa) palatine inferior nasal conchae vomer Relevant Figures: 7.4, 7.6, 7.7a Sutures coronal sagittal lambdoid squamous Relevant Figures: 7.4, 7.5 Paranasal Sinuses frontal sphenoidal maxillary ethmoidal Relevant Figures: 7.15 Fontanels anterior posterior sphenoidal mastoid Relevant Figures: 7.28 Hyoid bone Relevant Figures: 7.17 1 Vertebrae Parts of a “typical vertebra” using thoracic as example body vertebral arch (pedicle, lamina, vertebral foramen) intervertebral foramen transverse process spinous process superior articular process inferior articular process Divisions of vertebral column cervical (transverse foramina) thoracic (transverse costal facet - for tubercle of rib, superior and inferior costal -

Pelvis & Thigh
Pelvis & Thigh 6 After meeting a stranger, you soon begin to palpate their piriformis Topographical Views 276 muscle (located deep in the posterior buttock). You certainly wouldn’t try Exploring the Skin and Fascia 277 this in “everyday life,” but in patient care settings this level of familiarity is Bones of the Pelvis and Thigh 278 commonplace—and welcomed by a client with a hypercontracted piriformis. Bony Landmarks of the Pelvis Touch is a unique privilege afforded to health care providers. As such, we and Thigh 279 need to be mindful of the trust our clients have in us. One way to insure this Overview: Bony Landmark Trails 284 is through good communication skills. For instance, working the adductors Overview: Muscles of the and gluteal region requires a practitioner to provide ample explanation as to Pelvis and Thigh 296 the rationale, need, and goals of working these intimate areas of the body. Synergists—Muscles Working This chapter might pose new challenges for you, as we will be palpating Together 302 structures close to intimate areas. Muscles of the Pelvis and Thigh 306 Ligaments and Other Before proceeding, consider the following questions: Structures of the Pelvis and Thigh 336 E Have you ever been anxious to undergo a physical exam? Was there anything the practitioner did or could have done to alleviate this anxiety? Consider multiple elements, including both verbal and nonverbal communication, draping, physical pressure, and pace. E Tissues and landmarks found in the pelvis and thigh tend to be significantly larger than those discussed in previous chapters. How might your palpation techniques need to change? E Also, how might you properly and comfortably position your patient to access structures needing to be palpated. -

Morphological Variation of the Proximal Femur in Selected Skeletal Remains
MORPHOLOGICAL VARIATION OF THE PROXIMAL FEMUR IN SELECTED SKELETAL REMAINS A Thesis by Jessica Lynn Brown B.S. Michigan State University, 2002 Submitted to the College of Liberal Arts and Sciences and the faculty of the Graduate School of Wichita State University in partial fulfillment of the requirements for the degree of Master of Arts May 2006 MORPHOLOGICAL VARIATION OF THE PROXIMAL FEMUR IN SELECTED SKELETAL REMAINS I have examined the final copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Arts with a major in Anthropology. __________________________________________ Peer H. Moore-Jansen, Ph.D., Committee Chair We have read this thesis and recommend its acceptance: __________________________________________ Robert Lawless, Ph.D., Committee Member __________________________________________ John Carter, Ph.D., Committee Member ii DEDICATION To My Family and Friends iii ACKNOWLEDGEMENTS Funding for this research came from three generous sources. Dr. Todd Fenton provided me with the opportunity to experience Albania’s past and present in two separate field seasons. The Moore-Jansen Scholarship and the Nancy Berner Fund through the Anthropology Department at Wichita State University provided financial support to study the Hamann-Todd collection in Cleveland. The expense of travel and accommodations during data collection during all of these experiences was greatly appreciated by these considerate awards. I would like to thank all the members of my thesis committee, Dr. Peer Moore-Jansen, Dr. Robert Lawless, and Dr. John Carter for their comments and suggestions. Throughout this process Dr. Peer Moore-Jansen provided invaluable guidance on how to conduct and report meaningful research, which will stay with me always. -
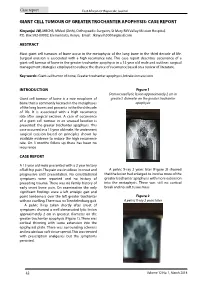
Giant Cell Tumour of Greater Trochanter Apophysis: Case Report
Case report East African Orthopaedic Journal GIANT CELL TUMOUR OF GREATER TROCHANTER APOPHYSIS: CASE REPORT Kinyanjui JW, MBChB, MMed (Orth), Orthopaedic Surgeon, St Mary Rift Valley Mission Hospital, P.O. Box 582-00902, Elementaita, Kenya. Email: [email protected] ABSTRACT Most giant cell tumours of bone occur in the metaphysic of the long bone in the third decade of life. Surgical excision is associated with a high recurrence rate. This case report describes occurrence of a giant cell tumour of bone in the greater trochanter apophysis in a 15 year old male and outlines surgical management strategies employed to reduce the chance of recurrence based on a review of literature. Key words: Giant cell tumor of bone, Greater trochanter apophysis, Intralesion excision INTRODUCTION Figure 1Figure 1 Demarcated lytic lesion approximately 2 cm in Giant cell tumour of bone is a rare neoplasm of greatest Figure diameterDemarcated 1 on the lytic greater lesion trochanter approximately 2 cm in greatest bone that is commonly located in the metaphyses diameterapophysis on the greater trochanter apophysis of the long bones and presents in the third decade Demarcated lytic lesion approximately 2 cm in greatest of life. It is associated with a high recurrence diameter on the greater trochanter apophysis rate after surgical excision. A case of occurrence of a giant cell tumour in an unusual location is presented: the greater trochanter apophysis. This case occurred in a 15 year old male. He underwent surgical excision based on principles shown by available evidence to reduce the high recurrence rate. On 3 months follow up there has been no recurrence. -

The Third Trochanter of Femur - an Anatomical Study
International Journal of Current Research and Review Research Article DOI: http://dx.doi.org/10.31782/IJCRR.2020.122333 The Third Trochanter of Femur - An Anatomical Study IJCRR 1 1 2 Section: Healthcare Gyanaranjan Nayak , Saurjya Ranjan Das , Sitansu Kumar Panda , Sci. Journal Impact 3 Factor: 6.1 (2018) B Shanta Kumari ICV: 90.90 (2018) 1Associate Professor, Department of Anatomy, IMS and SUM Hospital, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, 2 Copyright@IJCRR Odisha 751003, India; Professor, Department of Anatomy, IMS and SUM Hospital, Siksha ‘O’ Anusandhan Deemed to be University, Bhu- baneswar, Odisha 751003, India; 3Assistant Professor, Department of Anatomy, IMS and SUM Hospital, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, Odisha 751003, India. ABSTRACT . Background: The third trochanter is a rounded, linear or conical projection along the superior border of gluteal tuberosity of the femur. Purpose of the study is to find the frequency of the third trochanter in human femora and determine its dimensions and its distance from the tip of greater trochanter of the femur. Methods: Sixty dry human femora (thirty right and thirty left) were examined for the presence of third trochanter, their dimen- sions and their distance from greater trochanter being measured by slide calliper. Results: The third trochanter was noted in 21.66 % cases. Their mean length and width were found to be 2.61 ± 0.97 cm and 0.96 ± 0.15 cm respectively. The average distance of the third trochanter from the tip of the greater trochanter was found to be 6.81 ± 0.64 cm. Conclusion: The current study will be useful in anthropometry and orthopaedics. -

Medd 421 Anatomy Project ~
MEDD 421 ANATOMY PROJECT ~ KURT MCBURNEY, ASSISTANT TEACHING PROFESSOR - IMP NICHOLAS BYERS - SMP PETER BAUMEISTER - SMP Proof of Permission for Cadaveric Photos LABORATORY 1 ~ OSTEOLOGY INDEX Acetabular labrum Gluteal surface Metatarsals (1-5) Acetabulum Greater sciatic notch Navicular Anterior intercondylar area Greater Trochanter Neck of Fibula Anterior superior iliac spine (ASIS) Head of Femur Neck of Talus Calcaneal Tuberosity Head of Fibula Obturator foramen Calcaneus Head of Talus Patellar Surface Cuboid Iliac crest Phalanges Cuneiform Intercondylar eminence Phalanges (medial, intermediate, and lateral) Ischial spine Posterior superior iliac spine (PSIS) Femoral Condyles Ischial tuberosity Round ligament of the head of the femur Femoral Epicondyles Lateral Malleolus Shaft Fovea Capitis Lesser sciatic notch Sustentaculum tali Neck of Femur Linea Aspera Talus Gerdy’s tubercle Lunate surface Tarsus Medial / Lateral Tibial Condyles Tibial tuberosity Medial Malleolus Trochlear surface OSTEOLOGY: THE FOOT Structures in View: Calcaneus Talus Cuboid Navicular Cuneiform (Medial specific) Metatarsals (5th specific) Phalanges Calcaneus Structures in View: Sustentaculum Tali Calcaneal Tuberosity (Insertion of Achilles) Talus Structures in View: Head Neck Trochlear Surface (Not the spring) Metatarsals Structures in View: Head Shaft Base First Metatarsal Fifth Metatarsal Phalanges Structures in View: Proximal Distal Proximal Middle Distal Femur (anterior) Structures in View: Patellar Surface Medial Epicondyle Lateral Epicondyle Medial Condyle