I SPATIAL HABITAT VARIATION in a GREAT PLAINS RIVER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution and Growth of Blue Sucker in a Great Plains River, USA
Fisheries Management and Ecology, 2007, 14, 255–262 Distribution and growth of blue sucker in a Great Plains river, USA J. L. EITZMANN & A. S. MAKINSTER* Division of Biology, Kansas State University, Manhattan, KS, USA C. P. PAUKERT U.S. Geological Survey, Kansas Cooperative Fish and Wildlife Research Unit, Division of Biology, Kansas State University, Manhattan, KS, USA Abstract Blue sucker, Cycleptus elongatus (Le Sueur), was sampled in the Kansas River, Kansas, USA to determine how relative abundance varies spatially and growth compares to other populations. Electric fishing was conducted at 36 fixed sites during five time periods from March 2005 to January 2006 to determine seasonal distribution. An additional 302 sites were sampled in summer 2005 to determine distribution throughout the river. A total of 101 blue sucker was collected ranging from 242 to 782 mm total length and 1–16 years old. Higher catch rates were observed in upper river segments and below a low-head dam in lower river segments, and catch rates were higher during November in the upriver sites. Kansas River blue sucker exhibited slower growth rates than other populations in the Great Plains including populations as far north as South Dakota. KEYWORDS: Blue sucker, Cycleptus elongatus, Kansas River. reducing preferred habitat (Tomelleri & Eberle 1990; Introduction Pflieger 1997; Vokoun et al. 2003). Although studies Blue sucker, Cycleptus elongatus (Le Sueur), is distri- have focused on blue sucker spawning events (Vokoun buted throughout the Mississippi and Missouri river et al. 2003), no studies to our knowledge have evalu- drainages, USA. Its range extends from Montana ated the distribution, abundance and habitat use of south to Mexico, and east to Pennsylvania (Moss, blue sucker throughout a large river across several Scanlan & Anderson 1983; Morey & Berry 2003; seasons. -

Kansas River Basin Model
Kansas River Basin Model Edward Parker, P.E. US Army Corps of Engineers Kansas City District KANSAS CITY DISTRICT NEBRASKA IOWA RATHBUN M I HARLAN COUNTY S S I LONG S S I SMITHVILLE BRANCH P TUTTLE P CREEK I URI PERRY SSO K MI ANS AS R I MILFORD R. V CLINTON E WILSON BLUE SPRINGS R POMONA LONGVIEW HARRY S. TRUMAN R COLO. KANOPOLIS MELVERN HILLSDALE IV ER Lake of the Ozarks STOCKTON KANSAS POMME DE TERRE MISSOURI US Army Corps of Engineers Kansas City District Kansas River Basin Operation Challenges • Protect nesting Least Terns and Piping Plovers that have taken residence along the Kansas River. • Supply navigation water support for the Missouri River. • Reviewing requests from the State of Kansas and the USBR to alter the standard operation to improve support for recreation, irrigation, fish & wildlife. US Army Corps of Engineers Kansas City District Model Requirements • Model Period 1/1/1920 through 12/31/2000 • Six-Hour routing period • Forecast local inflow using recession • Use historic pan evaporation – Monthly vary pan coefficient • Parallel and tandem operation • Consider all authorized puposes • Use current method of flood control US Army Corps of Engineers Kansas City District Model PMP Revisions • Model period from 1/1/1929 through 12/30/2001 • Mean daily flows for modeling rather than 6-hour data derived from mean daily flow values. • Delete the requirement to forecast future hydrologic conditions. • Average monthly lake evaporation rather than daily • Utilize a standard pan evaporation coefficient of 0.7 rather than a monthly varying value. • Separate the study basin between the Smoky River Basin and the Republican/Kansas River Basin. -

The 1951 Kansas - Missouri Floods
The 1951 Kansas - Missouri Floods ... Have We Forgotten? Introduction - This report was originally written as NWS Technical Attachment 81-11 in 1981, the thirtieth anniversary of this devastating flood. The co-authors of the original report were Robert Cox, Ernest Kary, Lee Larson, Billy Olsen, and Craig Warren, all hydrologists at the Missouri Basin River Forecast Center at that time. Although most of the original report remains accurate today, Robert Cox has updated portions of the report in light of occurrences over the past twenty years. Comparisons of the 1951 flood to the events of 1993 as well as many other parenthetic remarks are examples of these revisions. The Storms of 1951 - Fifty years ago, the stage was being set for one of the greatest natural disasters ever to hit the Midwest. May, June and July of 1951 saw record rainfalls over most of Kansas and Missouri, resulting in record flooding on the Kansas, Osage, Neosho, Verdigris and Missouri Rivers. Twenty-eight lives were lost and damage totaled nearly 1 billion dollars. (Please note that monetary damages mentioned in this report are in 1951 dollars, unless otherwise stated. 1951 dollars can be equated to 2001 dollars using a factor of 6.83. The total damage would be $6.4 billion today.) More than 150 communities were devastated by the floods including two state capitals, Topeka and Jefferson City, as well as both Kansas Cities. Most of Kansas and Missouri as well as large portions of Nebraska and Oklahoma had monthly precipitation totaling 200 percent of normal in May, 300 percent in June, and 400 percent in July of 1951. -

IRRIGATION-WELL DEVELOPMENT in the KANSAS RIVER BASIN of EASTERN COLORADO UNITED STATES DEPARTMENT of the INTERIOR Douglas Mckay, Secretary
GEOLOGICAL SURVEY CIRCULAR 295 IRRIGATION-WELL DEVELOPMENT IN THE KANSAS RIVER BASIN OF EASTERN COLORADO UNITED STATES DEPARTMENT OF THE INTERIOR Douglas McKay, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director GEOLOGICAL SURVEY CIRCULAR 295 IRRIGATION-WELL DEVELOPMENT IN THE KANSAS RIVER BASIN OF EASTERN COLORADO By W. D. E. Cardwell Washington, D. C.,1953 Free on application to the Geological Snrvey, Washington 25, D. C. CONTENTS Page Page Abstract............................... 1 Use of ground water Continued Introduction........................... 2 Possibilities for development of Purpose of the investigation......... 2 irrigation from wells ............. 12 Location and extent of the region.... 2 Munic ipal water supplies ........... 13 Methods of investigation............. 2 Akron............................ We11-numbering system................ 3 Arriba .......................... Acknowledgments...................... h Bethune......................... Geography.............................. k Burlington...................... Topography and drainage.............. h Cheyenne Wells.................. Climate.............................. 5 Eckley.......................... Geology................................ 7 Flagler......................... 15 Stratigraphy......................... 7 Fleming......................... 15 Geologic formations and their water Genoa........................... 15 bearing properties.................. 7 Haxtun.......................... 15 Cretaceous system.................. 7 Holyoke........................ -

JOHNSON 3 3 R 14 COUNTY 16 E 167TH ST 14 Clare IV RS 1638 RS L INDUSTRIA 16 Ek 16 15 17 14 13 Re R 4 2 22 38°50' 18 1 7 Four AIRPORT C 3 2 3 6 3 14 13 13 56 6 N Em
LEGEND ROADS AND ROADWAY FEATURES Controlled Access (With Interchange) - Interstate CONSERVATION AND RECREATION Kansas Turnpike (KTA) (With Interchange) US Route - Controlled Access (With Interchange) Public Recreation US Route - Divided Scenic, Tourist, or Historical Site US Route - Undivided Trailer Park State Route - Controlled Access (With Interchange) Hotel or Motel State Route - Divided Camp or Lodge (Permanent Site with Building) State Route - Undivided Small Park ( SP - State Park, CP - County Park, RS Route - Divided MP = Municipal Park, SR - Safety Rest Area) RS Route - Paved Fish Hatchery RS Route - Unpaved Game Farm ' 0 Minor Road - Paved Game Preserve or Bird Sanctuary 5 ° 4 Minor Road - Stone or Gravel Rifle Club (Public) 9 Minor Road - Soil Golf Course or Country Club T Side Road or Street in Unincorporated Area Riding Academy, Saddle Club, or Stables 4 O I 30 29 3 - J 5 7 C Race Course or Speedway Ch/Cem. 6 0 T 0 ROAD SYSTEM DESIGNATION 25 14 . KUMP . 2 16 . 2 2 2 W 3 16 5 AVE. T Marina . O S . 4 30 RS T . Rural Secondary System . T O . S S W ' Y S K T . T D 3 T Rodeo Grounds . A O H 3 K V . S 2 0 . OA GRO E RD I 32 H N . E T 0 5 E H E C 2 E E 0 5 2. S C B T 1 1 2 T 2 . 3 s T 4 A 3 D 1 T N J H t P 7 7 3 S 0 8 . C 3 S . t 6 C N 3 F 4 4 8 Y 4 O D V . -

Kansas River Wildlife Area Access at This Time
he Kansas River Wildlife Area access at this time. Once complete of farmland and riparian corridors. ansas consists of four different the Riley County tract will provide Special use restrictions are in place KK Ttracts in Shawnee, Riley and an additional motor boat access site for all properties. Please check the Geary Counties all adjacent to the to the river near Ogden as well as area kiosk or with the Topeka Kansas River. The Riley and Geary walk-in access to 300 acres of river Regional office for these restrictions County tracts are currently under bottom ground. The two Shawnee or questions. development and not open to public County tracts include over 200 acres RRiver HUNTING THINGS TO REMEMBER Wildlife Area The two Shawnee County tracts (Urish Road & Mac A safety zone has been established on this tract that Vicar Road) are managed independently and each have prohibits the discharge of any firearm. This will be strict- unique access and hunting restrictions. ly enforced and all violations will be cited. Hunting pres- The Mac Vicar Road tract is located within the City lim- sure can be very high during the dove season. Hunters its of Topeka and is only open to archery hunting through should utilize safe hunting techniques and be aware of the Department’s special hunts program. You can check their surroundings at all times. online at kdwp.state.ks.us to view these opportunities. The Urish Road tract is located on the north end of Kansas River WA NEBRASKA Urish Road, is adjacent to the City limits of Topeka and 159 7 NEMAHA BROWN 77 provides a variety of outdoor opportunities. -

Flood Control for the Missouri River Basin
By the KansasHistorical Society FloodControl for theMissouri River Basin The Missouri River,the longestriver in North America, drains two thirds of the Great Plainseast of the Rocky Mountains including parts of 10 states and two Canadian provinces.Many citieswith large population lie along the Missouri Riverincluding Kansas City and St. Louis. Damshave been built throughoutthe systemin an MissouriR iver basin. attemptto control flooding on the Missouri. The KansasRiver is one of the major tributariesof the Missouri.The Kansasand its tributariesdrain more than 60,000 square milesof land through Kansas,southern Nebraska, and easternColorado . The major tributaries of the KansasRiver are the Republican,Big Blue,and SmokyHill rivers. Throughouthistory the KansasRiver Valley has experiencedmajor flooding. Kansaand Osage villages along with some Indian missionswere affected in the 1844 flood. The next major flood didn't occur until 1903. By this time many cities had been establishedalong the river. Thisflood severelyimpacted the citizensof Manhattan, Topeka,Lawrence, and KansasCity. KansasRiver system. The first official mentionof building a dam near the mouthof the Big Blue Rivernorth of Manhattan occurred in 1928. The statedpurpose was flood control and conservationof water. No appropriations (provisionof money) for building the dam were included, so the project stayed on the drawing board. In 1935 the KansasRiver once again flooded. By this time the U. S. Army Corps of Engineershad becomethe lead federal flood control agency.The Corps recommendedthe building of sevendams and reservoirsthroughout the Missouri River, including TuttleCreek. Theseflood control dams would protect residentsand businessesalong the river as well as provide work for the hundredsof unemployedworkers during the Great Depression. -
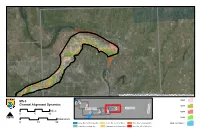
MS-3 Channel Alignment Dynamics
Source: Esri, DigitalGlobe, GeoEye, i-cubed, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, and the GIS User Community 1804 MS-3 6 Channel Alignment Dynamics A 5 N 1879 A MISSOURI 1 ILLINOIS I D Miles N 1894 4 3 2 I 0 5 10 er iv R e N ag Osage River 1928 s Kilometers O 0 10 20 Osage River to Mississippi River Kansas River to Grand River Platte River to Nodaway River Modern Channel Grand River to Osage River Nodaway River to Kansas River River Mile 670 to Platte River Source: Esri, DigitalGlobe, GeoEye, i-cubed, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, and the GIS User Community 1804 7 6 MS-3 5 Channel Alignment Dynamics 1 1879 ILLINOIS M4ISSOURI 2 Miles 3 er 1894 iv Osage River R 0 5 10 ge sa O N Osage River 1928 Kilometers 0 10 20 Osage River to Mississippi River Kansas River to Grand River Platte River to Nodaway River Modern Channel Grand River to Osage River Nodaway River to Kansas River River Mile 670 to Platte River Source: Esri, DigitalGlobe, GeoEye, i-cubed, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, and the GIS User Community 8 6 1804 MS-3 7 5 Channel Alignment Dynamics 1 3 1879 MISSOURI 2 ILLINOIS Miles e4r 1894 iv R 0 5 10 ge sa O N Osage River 1928 Kilometers 0 10 20 Osage River to Mississippi River Kansas River to Grand River Platte River to Nodaway River Modern Channel Grand River to Osage River Nodaway River to Kansas River River Mile 670 to Platte River Source: Esri, DigitalGlobe, GeoEye, i-cubed, USDA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, and the GIS User Community 8 6 1804 MS-3 7 5 ILLINOIS K Channel -

Quality of Streams in Johnson County, Kansas, and Relations to Environmental Variables, 2003–07
80 March 2007 60 3.0 Urban watershed 40 Fully supporting (greater than 2.49) Rural watershed 20 2.5 METRIC SCORES T OR P 2003 data P U 0 S 20 - 04 data Prepared in cooperation with the Johnson County Stormwater ManagementE Program F I 2 60 July 2007 L 007 data - C 2.0 I Partially supporting (1.5–2.49) T A 50 U Q A E H PERCENTAGE OF EUTRAPHENTIC DIATOMS D 40 K FOUR 1. 5 30 Y OF T I L Nonsupporting (1.0–1.49) A U Q 20 MEAN L A C I 10 G O 1.0 L O I IN1b B IN3a 0 IN6 LM 1a LM 1b LM 94°40’ 1c M IN3a Wyandotte County I1 M k I4 M iver 50’ 45’ e I7 T IN6 R re ek O2 IMPROVING e TU1 s C r SAMPLING SITE (fig. B1)I1 BI1 a y C BL s e ck 3 BL n k o 4 INCREASING DISTANCE FROMCE6 WASTEWATER DISCHARGE r k BL5 a R e C u e A1 K T r CE1 MI7 CE6 C KI5b h KI6 SAMPLING SITEKI6b (fig. 1) MI7 s b u TU1* TU1 r ar Creek LM1b B * IndicatesC nole wastewater effects TO2* Liittttlle Miillll C rree D Rural sites IN1b* ee yk LM1a kk es 94°55’ BL5* B ran CA1* LM1c ch 95°00’ Leavenworth County MI4 IN6 Urban sites 39°00’ 06893300 Creek CE6 n TO2 dia In kk 1,000 C ee IN3a e ee d r r k KI6b a IN1b e C C 2003 data r e l l r l l i C i C 900 r k 2004 data M e M w ek a h 2007 data MI1 a r m e o v 800 CA1 T i K R i ll 38°55’ CC Olathe 700 rr e e BL5 k k k e e e e 600 r r KI5b CE1 e C C u l B nn Coffe ii e Cr aa ee 500 tt k BL4 pp aa BL3 C Douglas County Douglas 400 KANSAS KANSAS eek MISSOURI Wolf Cr 50’ 300 L i t * Downstream from wastewater discharge (table 1) tl SCORE MACROINVERTEBRATE 10-METRIC BB e KI6b* ii B KI5b* gg 200 CE1 CE6* u l CA1 BB l BL5 BL4 uu C BL3 ll r WyandotteWyandotte ll e BI1* TU1 C e 100 TO2 k r Leavenworth CountyCounty MI7* e r e MI1 MI4*e Riiv SAMPLING SITE (fig. -

Surface-Water-Quality Assessment of the Lower Kansas River Basin
Surface-Water-Quality Assessment of the Lower Kansas River Basin, Kansas and Nebraska: Selected Metals, Arsenic, and Phosphorus in Streambed Sediments of First- and Second-Order Streams, 1987 By D.Q. TANNER and J.L RYDER U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 94-4196 Prepared as part of the NATIONAL WATER-QUALITY ASSESSMENT PROGRAM Lawrence, Kansas 1996 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY GORDON P. EATON, Director For additional information write to: Copies of this report can be purchased from: U.S. Geological Survey Earth Science Information Center District Chief Open-File Reports Section U.S. Geological Survey Box25286, MS 517 4821 Quail Crest Place Denver Federal Center Lawrence, Kansas 66049-3839 Denver, Colorado 80225 CONTENTS Abstract ....................................................................................................................................................................................1 Introduction...................................................................................................^ F^ipose and Scope............................................................... Description of Study Unit...'...........................................................................................................................................^ Methods of Data Collection and Analysis................................................................................................................................5 Selected Metals in Streambed -

Kansas River Trail
Kansas (KAW) River History • The first map of the Kansas River is dated back to 1718. • Lewis and Clark spent 3 days camped at Kaw Point at the confluence of the Kansas and Missouri Rivers. Kansas (KAW) River History • The Kaw was declared a navigable river in 1857 by the Kansas Legislature to encourage navigation. • The legislature repealed this law in 1864 at the encouragement of the railroad to reduce competition. • The river was restored to navigable status by the legislature in 1913. Kansas (KAW) River History . The Kaw has long been known for it’s fishing and recreational opportunities. Public access ramps for boat and canoe use were first funded by the state in the 1970’s. Background • There are only three navigable rivers in Kansas (Kansas, Ark & Missouri). All other streams are private in Kansas. • Kansas River is approximately174 miles long, extending from Junction City to Kansas City. • A few access sites were constructed in the 1970’s and early 1980’s on the Kansas River. • Renewed interest in the 1990’s, “Kansas River Recreation” plan was developed in 1998. • Goal was to place access every 10-15 miles. • In 2000, there were only 5 public access sites. Background Cont. • Over $1,000,000 has been committed by KDWPT to develop access since 2000. Local funds and donations have probably equaled this amount. • An additional 14 access sites have been constructed. Background Cont. • KDWPT was contacted in 2011 about potential projects that would fall within the America’s Great Outdoors program. • The Kansas River water trail project was identified as a potential project for this program. -

Historical Evidence of Riparian Forests in the Great Plains and How That Knowledge Can Aid with Restoration and Management
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Forest Service -- USDA Forest Service / UNL Faculty Publications National Agroforestry Center September 2004 Historical evidence of riparian forests in the Great Plains and how that knowledge can aid with restoration and management. Elliot West University of Arkansas in Fayetteville, Arkansas Greg Ruark U.S. Department of Agriculture’s National Agroforestry Center in Lincoln, Nebraska Follow this and additional works at: https://digitalcommons.unl.edu/usdafsfacpub Part of the Forest Sciences Commons West, Elliot and Ruark, Greg, "Historical evidence of riparian forests in the Great Plains and how that knowledge can aid with restoration and management." (2004). USDA Forest Service / UNL Faculty Publications. 7. https://digitalcommons.unl.edu/usdafsfacpub/7 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Forest Service -- National Agroforestry Center at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA Forest Service / UNL Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. a long, long time ago... Elliott West is a professor of history at the University of Arkansas in Fayetteville, Arkansas. Greg Ruark is the director of the U.S. Department of Agriculture’s National Agroforestry Center in Lincoln, Nebraska. Historical evidence of riparian forests in the Great Plains and how that knowledge can aid with restoration and management. Riparian areas—land adja- transcontinental railroad spur lines in the 1860’s; before the 1859 Denver gold cent to a streambank or rush; and before the Great Westward other water body—filtering Movement of the 1840’s along the nonpoint source pollution.