Managing Complications of Pleural Procedures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coding Billing
CodingCoding&Billing FEBRUARY 2020 Quarterly Editor’s Letter Welcome to the February issue of the ATS Coding and Billing Quarterly. There are several important updates about the final Medicare rules for 2020 that will be important for pulmonary, critical care and sleep providers. Additionally, there is discussion of E/M documentation rules that will be coming in 2021 that practices might need some time to prepare for, and as always, we will answer coding, billing and regulatory compliance questions submitted from ATS members. If you are looking for a more interactive way to learn about the 2020 Medicare final rules, there is a webinar on the ATS website that covers key parts of the Medicare final rules. But before we get to all this important information, I have a request for your help. EDITOR ATS Needs Your Help – Recent Invoices for Bronchoscopes and PFT Lab ALAN L. PLUMMER, MD Spirometers ATS RUC Advisor TheA TS is looking for invoices for recently purchased bronchoscopes and ADVISORY BOARD MEMBERS: PFT lab spirometer. These invoices will be used by theA TS to present practice KEVIN KOVITZ, MD expense cost equipment to CMS to help establish appropriate reimbursement Chair, ATS Clinical Practice Committee rates for physician services using this equipment. KATINA NICOLACAKIS, MD Member, ATS Clinical Practice Committee • Invoices should not include education or service contract as those ATS Alternate RUC Advisorr are overhead and cannot be considered by CMS for this portion of the STEPHEN P. HOFFMANN, MD Member, ATS Clinical Practice Committee formula and payment rates. ATS CPT Advisor • Invoices can be up to five years old. -

Mediastinitis and Bilateral Pleural Empyema Caused by an Odontogenic Infection
Radiol Oncol 2007; 41(2): 57-62. doi:10.2478/v10019-007-0010-0 case report Mediastinitis and bilateral pleural empyema caused by an odontogenic infection Mirna Juretic1, Margita Belusic-Gobic1, Melita Kukuljan3, Robert Cerovic1, Vesna Golubovic2, David Gobic4 1Clinic for Oral and Maxillofacial Surgery, 2Clinic for Anaesthesiology and Reanimatology, 3Department of Radiology, 4Clinic for Internal Medicine, Clinical hospital, Rijeka, Croatia Background. Although odontogenic infections are relatively frequent in the general population, intrathoracic dissemination is a rare complication. Acute purulent mediastinitis, known as descending necrotizing mediastin- itis (DNM), causes high mortality rate, even up to 40%, despite high efficacy of antibiotic therapy and surgical interventions. In rare cases, unilateral or bilateral pleural empyema develops as a complication of DNM. Case report. This case report presents the treatment of a young, previously healthy patient with medias- tinitis and bilateral pleural empyema caused by an odontogenic infection. After a neck and pharynx re-inci- sion, and as CT confirmed propagation of the abscess to the thorax, thoracotomy was performed followed by CT-controlled thoracic drainage with continued antibiotic therapy. The patient was cured, although the recognition of these complications was relatively delayed. Conclusions. Early diagnosis of DNM can save the patient, so if this severe complication is suspected, thoracic CT should be performed. Key words: mediastinitis; empyema, pleural; periapical abscess – complications Introduction rare complication of acute mediastinitis.1-6 Clinical manifestations of mediastinitis are Acute suppurative mediastinitis is a life- frequently nonspecific. If the diagnosis of threatening infection infrequently occur- mediastinitis is suspected, thoracic CT is ring as a result of the propagation of required regardless of negative chest X-ray. -

PICTORIAL REVIEW Thoracic Involvement in Connective
JBR–BTR, 2015, 98: 3-19. PICTORIAL REVIEW THORACIC INVOLVEMENT IN CONNECTIVE TISSUE DISEASES: RADIO- LOGICAL PATTERNS AND FOLLOW-UP G. Serra1, A.-L. Brun1, P. Ialongo2, M.-L. Chabi1, P.A. Grenier1 Connective tissue diseases (CTDs) are a heterogeneous group of idiopathic inflammatory diseases involving various organs. A thoracic involvement is frequent, and chest-CT represents the imaging technique of reference in its assess- ment. Pulmonary abnormalities related to CTDs are various; although several disease-specific aspects have been described, the two most clinically relevant complications are represented by interstitial lung disease and pulmonary arterial hypertension. The early identification of a thoracic involvement, with the adoption of specific therapies, can significantly change patient’s prognosis. The aim of this article is to review the most common typical and atypical CT features of thoracic involvement occurring in CT, especially focusing on interstitial lung disease. Key-word: Connective tissue, diseases – Lung, interstitial disease – Hypertension, pulmonary. Connective tissue diseases (CTDs) at the time of diagnosis of CTD, or chronic inflammation in the alveolar are a heterogeneous group of in- more commonly manifest later in the walls. The patients usually response flammatory diseases derived from course of the disease (5, 6). well to corticosteroid therapy and an immunologic deregulation affect- The most common histopatholog- have a good prognosis. However pa- ing various organs. A thoracic in- ic patterns of ILD seen in the setting tients with OP associated with CTD volvement (pulmonary, pleural or of CTDs are non specific interstitial seem to have a greater tendency to mediastinal) can be frequently pneumonia (NSIP), usual interstitial develop fibrosis and a higher mortal- found; its frequency and expression pneumonia (UIP), organizing pneu- ity than patients with cryptogenic depends on the type of disease, and monia (OP), diffuse alveolar damage OP (5, 6). -

Thoracentesis
The new england journal of medicine videos in clinical medicine Thoracentesis Todd W. Thomsen, M.D., Jennifer DeLaPena, M.D., and Gary S. Setnik, M.D. INDICATIONS From the Department of Emergency Medi- Thoracentesis is a valuable diagnostic procedure in a patient with pleural effusion cine, Mount Auburn Hospital, Cambridge, of unknown causation. Analysis of the pleural fluid will allow its categorization as MA (T.W.T., G.S.S.); the Department of Emergency Medicine, Beth Israel Deacon- either a transudate (a product of unbalanced hydrostatic forces) or an exudate (a ess Medical Center, Boston (J.D.); and the product of increased capillary permeability or lymphatic obstruction) (Table 1). If Division of Emergency Medicine, Harvard the effusion seems to have an obvious source (e.g., in an afebrile patient with con- Medical School, Boston (T.W.T., J.D., G.S.S.). Address reprint requests to Dr. Thomsen gestive heart failure and bilateral pleural effusions), diagnostic thoracentesis may at the Department of Emergency Medi- be deferred while the underlying process is treated. The need for the procedure cine, Mount Auburn Hospital, 330 Mount should be reconsidered if there is no appropriate response to therapy.1 Auburn St., Cambridge, MA 02238, or at [email protected]. Thoracentesis, as a therapeutic procedure, may dramatically reduce respiratory distress in patients presenting with large effusions. N Engl J Med 2006;355:e16. Copyright © 2006 Massachusetts Medical Society. CONTRAINDICATIONS There are limited data on the safety of thoracentesis -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -
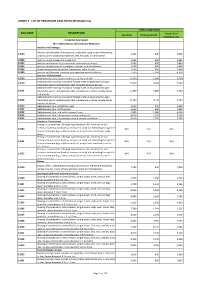
Annex 2. List of Procedure Case Rates (Revision 2.0)
ANNEX 2. LIST OF PROCEDURE CASE RATES (REVISION 2.0) FIRST CASE RATE RVS CODE DESCRIPTION Health Care Case Rate Professional Fee Institution Fee Integumentary System Skin, Subcutaneous and Accessory Structures Incision and Drainage Incision and drainage of abscess (e.g., carbuncle, suppurative hidradenitis, 10060 3,640 840 2,800 cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia) 10080 Incision and drainage of pilonidal cyst 3,640 840 2,800 10120 Incision and removal of foreign body, subcutaneous tissues 3,640 840 2,800 10140 Incision and drainage of hematoma, seroma, or fluid collection 3,640 840 2,800 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst 3,640 840 2,800 10180 Incision and drainage, complex, postoperative wound infection 5,560 1,260 4,300 Excision - Debridement 11000 Debridement of extensive eczematous or infected skin 10,540 5,040 5,500 Debridement including removal of foreign material associated w/ open 11010 10,540 5,040 5,500 fracture(s) and/or dislocation(s); skin and subcutaneous tissues Debridement including removal of foreign material associated w/ open 11011 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 11,980 5,880 6,100 and muscle Debridement including removal of foreign material associated w/ open 11012 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 12,120 6,720 5,400 muscle, and bone 11040 Debridement; skin, partial thickness 3,640 840 2,800 11041 Debridement; skin, full thickness 3,640 840 2,800 11042 Debridement; skin, and -

ANMC Specialty Clinic Services
Cardiology Dermatology Diabetes Endocrinology Ear, Nose and Throat (ENT) Gastroenterology General Medicine General Surgery HIV/Early Intervention Services Infectious Disease Liver Clinic Neurology Neurosurgery/Comprehensive Pain Management Oncology Ophthalmology Orthopedics Orthopedics – Back and Spine Podiatry Pulmonology Rheumatology Urology Cardiology • Cardiology • Adult transthoracic echocardiography • Ambulatory electrocardiology monitor interpretation • Cardioversion, electrical, elective • Central line placement and venous angiography • ECG interpretation, including signal average ECG • Infusion and management of Gp IIb/IIIa agents and thrombolytic agents and antithrombotic agents • Insertion and management of central venous catheters, pulmonary artery catheters, and arterial lines • Insertion and management of automatic implantable cardiac defibrillators • Insertion of permanent pacemaker, including single/dual chamber and biventricular • Interpretation of results of noninvasive testing relevant to arrhythmia diagnoses and treatment • Hemodynamic monitoring with balloon flotation devices • Non-invasive hemodynamic monitoring • Perform history and physical exam • Pericardiocentesis • Placement of temporary transvenous pacemaker • Pacemaker programming/reprogramming and interrogation • Stress echocardiography (exercise and pharmacologic stress) • Tilt table testing • Transcutaneous external pacemaker placement • Transthoracic 2D echocardiography, Doppler, and color flow Dermatology • Chemical face peels • Cryosurgery • Diagnosis -

Differentiation of Lung Cancer, Empyema, and Abscess Through the Investigation of a Dry Cough
Open Access Case Report DOI: 10.7759/cureus.896 Differentiation of Lung Cancer, Empyema, and Abscess Through the Investigation of a Dry Cough Brittany Urso 1 , Scott Michaels 1, 2 1. College of Medicine, University of Central Florida 2. FM Medical, Inc. Corresponding author: Brittany Urso, [email protected] Abstract An acute dry cough results commonly from bronchitis or pneumonia. When a patient presents with signs of infection, respiratory crackles, and a positive chest radiograph, the diagnosis of pneumonia is more common. Antibiotic failure in a patient being treated for community-acquired pneumonia requires further investigation through chest computed tomography. If a lung mass is found on chest computed tomography, lung empyema, abscess, and cancer need to be included on the differential and managed aggressively. This report describes a 55-year-old Caucasian male, with a history of obesity, recovered alcoholism, hypercholesterolemia, and hypertension, presenting with an acute dry cough in the primary care setting. The patient developed signs of infection and was found to have a lung mass on chest computed tomography. Treatment with piperacillin-tazobactam and chest tube placement did not resolve the mass, so treatment with thoracotomy and lobectomy was required. It was determined through surgical investigation that the patient, despite having no risk factors, developed a lung abscess. Lung abscesses rarely form in healthy middle-aged individuals making it an unlikely cause of the patient's presenting symptom, dry cough. The patient cleared his infection with proper management and only suffered minor complications of mild pneumoperitoneum and pneumothorax during his hospitalization. Categories: Cardiac/Thoracic/Vascular Surgery, Infectious Disease, Pulmonology Keywords: lung abscess, empyema, lung infection, pneumonia, thoracotomy, lobectomy, pulmonology, respiratory infections Introduction Determining the etiology of an acute dry cough can be an easy diagnosis such as bronchitis or pneumonia; however, it can also develop from other etiologies. -

Management of Malignant Pleural Effusions an Official ATS/STS/STR Clinical Practice Guideline David J
AMERICAN THORACIC SOCIETY DOCUMENTS Management of Malignant Pleural Effusions An Official ATS/STS/STR Clinical Practice Guideline David J. Feller-Kopman*, Chakravarthy B. Reddy*, Malcolm M. DeCamp, Rebecca L. Diekemper, Michael K. Gould, Travis Henry, Narayan P. Iyer, Y. C. Gary Lee, Sandra Z. Lewis, Nick A. Maskell, Najib M. Rahman, Daniel H. Sterman, Momen M. Wahidi, and Alex A. Balekian; on behalf of the American Thoracic Society, Society of Thoracic Surgeons, and Society of Thoracic Radiology THIS OFFICIAL CLINICAL PRACTICE GUIDELINE WAS APPROVED BY THE AMERICAN THORACIC SOCIETY OCTOBER 2018, THE SOCIETY OF THORACIC SURGEONS JUNE 2018, AND THE SOCIETY OF THORACIC RADIOLOGY JULY 2018 Background: This Guideline, a collaborative effort from the MPE; 3) using either an indwelling pleural catheter (IPC) or American Thoracic Society, Society of Thoracic Surgeons, and chemical pleurodesis in symptomatic patients with MPE and Society of Thoracic Radiology, aims to provide evidence-based suspected expandable lung; 4) performing large-volume recommendations to guide contemporary management of patients thoracentesis to assess symptomatic response and lung expansion; with a malignant pleural effusion (MPE). 5) using either talc poudrage or talc slurry for chemical pleurodesis; 6) using IPC instead of chemical pleurodesis in patients with Methods: A multidisciplinary panel developed seven questions nonexpandable lung or failed pleurodesis; and 7) treating using the PICO (Population, Intervention, Comparator, and IPC-associated infections with antibiotics and not removing the Outcomes) format. The GRADE (Grading of Recommendations, catheter. Assessment, Development and Evaluation) approach and the Evidence to Decision framework was applied to each question. Recommendations Conclusions: These recommendations, based on the best available were formulated, discussed, and approved by the entire panel. -

Respiratory Function Changes After Asbestos Pleurisy
Thorax: first published as 10.1136/thx.35.1.31 on 1 January 1980. Downloaded from Thorax, 1980, 35, 31-36 Respiratory function changes after asbestos pleurisy P H WRIGHT, A HANSON, L KREEL, AND L H CAPEL From the Cardiothoracic Institute, London Chest Hospital, East Ham Chest Clinic, London, and the Division of Radiology, Clinical Research Centre, Northwick Park Hospital, Harrow ABSTRACT Six patients with radiographic evidence of diffuse pleural thickening after industrial asbestos exposure are described. Five had computed tomography of the thorax. All the scans showed marked circumferential pleural thickening often with calcification, and four showed no significant evidence of intrapulmonary fibrosis (asbestosis). Lung function testing showed reduc- tion of the inspiratory capacity and the single-breath carbon monoxide transfer factor (TLco). The transfer coefficient, calculated as the TLCO divided by the alveolar volume determined by helium dilution during the measurement of TLco, was increased. Pseudo-static compliance curves showed markedly more negative intrapleural pressures at all lung volumes than found in normal people. These results suggest that the circumferential pleural thickening was preventing normal lung expansion despite abnormally great distending pressures. The pattern of lung function tests is sufficiently distinctive for it to be recognised in clinical practice, and suggests that the lungs are held rigidly within an abnormal pleura. The pleural thickening in our patients may have been related to the condition described as "benign asbestos pleurisy" rather than the copyright. interstitial fibrosis of asbestosis. Malignant pleural effusion associated with meso- necessary in many of these early cases, and opera- thelioma or bronchial carcinoma is a recognised tion notes recorded the presence of marked http://thorax.bmj.com/ complication of asbestos exposure. -

Occupational Lung Diseases
24 Occupational lung diseases Introduction i Occupational diseases are often thought to be Key points uniquely and specifically related to factors in the work environment; examples of such diseases are • Systematic under-reporting and the pneumoconioses. However, in addition to other difficulties in attributing causation both contribute to underappreciation of the factors (usually related to lifestyle), occupational burden of occupational respiratory exposures also contribute to the development or diseases. worsening of common respiratory diseases, such • Work-related exposures are estimated as chronic obstructive pulmonary disease (COPD), to account for about 15% of all adult asthma and lung cancer. asthma cases. • Boththe accumulation of toxic dust in the Information about the occurrence of occupational lungs and immunological sensitisation respiratory diseases and their contribution to to inhaled occupational agents can morbidity and mortality in the general population is cause interstitial lung disease. provided by different sources of varying quality. Some • Despite asbestos use being phased European countries do not register occupational out, mesothelioma rates are forecast diseases and in these countries, information about to continue rising owing to the long latency of the disease. the burden of such diseases is completely absent. • The emergence of novel occupational In others, registration is limited to cases where causes of respiratory disease in compensation is awarded, which have to fulfil specific recent years emphasises the need for administrative or legal criteria as well as strict continuing vigilance. medical criteria; this leads to biased information and underestimation of the real prevalence. Under- reporting of occupational disease is most likely to occur in older patients who are no longer at work but whose condition may well be due to their previous job. -
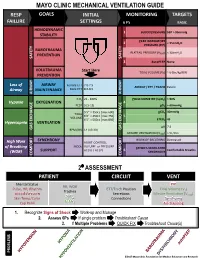
Mechanical Ventilation Guide
MAYO CLINIC MECHANICAL VENTILATION GUIDE RESP GOALS INITIAL MONITORING TARGETS FAILURE SETTINGS 6 P’s BASIC HEMODYNAMIC 1 BLOOD PRESSURE SBP > 90mmHg STABILITY PEAK INSPIRATORY 2 < 35cmH O PRESSURE (PIP) 2 BAROTRAUMA PLATEAU PRESSURE (P ) < 30cmH O PREVENTION PLAT 2 SAFETY SAFETY 3 AutoPEEP None VOLUTRAUMA Start Here TIDAL VOLUME (V ) ~ 6-8cc/kg IBW PREVENTION T Loss of AIRWAY Female ETT 7.0-7.5 AIRWAY / ETT / TRACH Patent Airway MAINTENANCE Male ETT 8.0-8.5 AIRWAY AIRWAY FiO2 21 - 100% PULSE OXIMETRY (SpO2) > 90% Hypoxia OXYGENATION 4 PEEP 5 [5-15] pO2 > 60mmHg 5’5” = 350cc [max 600] pCO2 40mmHg TIDAL 6’0” = 450cc [max 750] 5 VOLUME 6’5” = 500cc [max 850] ETCO2 45 Hypercapnia VENTILATION pH 7.4 GAS GAS EXCHANGE BPM (RR) 14 [10-30] GAS EXCHANGE MINUTE VENTILATION (VMIN) > 5L/min SYNCHRONY WORK OF BREATHING Decreased High Work ASSIST CONTROL MODE VOLUME or PRESSURE of Breathing PATIENT-VENTILATOR AC (V) / AC (P) 6 Comfortable Breaths (WOB) SUPPORT SYNCHRONY COMFORT COMFORT 2⁰ ASSESSMENT PATIENT CIRCUIT VENT Mental Status PIP RR, WOB Pulse, HR, Rhythm ETT/Trach Position Tidal Volume (V ) Trachea T Blood Pressure Secretions Minute Ventilation (V ) SpO MIN Skin Temp/Color 2 Connections Synchrony ETCO Cap Refill 2 Air-Trapping 1. Recognize Signs of Shock Work-up and Manage 2. Assess 6Ps If single problem Troubleshoot Cause 3. If Multiple Problems QUICK FIX Troubleshoot Cause(s) PROBLEMS ©2017 Mayo Clinic Foundation for Medical Education and Research CAUSES QUICK FIX MANAGEMENT Bleeding Hemostasis, Transfuse, Treat cause, Temperature control HYPOVOLEMIA Dehydration Fluid Resuscitation (End points = hypoxia, ↑StO2, ↓PVI) 3rd Spacing Treat cause, Beware of hypoxia (3rd spacing in lungs) Pneumothorax Needle D, Chest tube Abdominal Compartment Syndrome FLUID Treat Cause, Paralyze, Surgery (Open Abdomen) OBSTRUCTED BLOOD RETURN Air-Trapping (AutoPEEP) (if not hypoxic) Pop off vent & SEE SEPARATE CHART PEEP Reduce PEEP Cardiac Tamponade Pericardiocentesis, Drain.