Analysis of Key Genes and Pathways Associated with the Pathogenesis of Type 2 Diabetes Mellitus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Translational Control in Cancer Etiology
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Translational Control in Cancer Etiology Davide Ruggero Helen Diller Cancer Center, School of Medicine, University of California, San Francisco, California 94158 Correspondence: [email protected] The link between perturbations in translational control and cancer etiology is becoming a primary focus in cancer research. It has now been established that genetic alterations in several components of the translational apparatus underlie spontaneous cancers as well as an entire class of inherited syndromes known as “ribosomopathies” associated with in- creased cancer susceptibility. These discoveries have illuminated the importance of dereg- ulations in translational control to very specific cellular processes that contribute to cancer etiology. In addition, a growing body of evidence supports the view that deregulation of translational control is a common mechanism by which diverse oncogenic pathways promote cellular transformation and tumor development. Indeed, activation of these key oncogenic pathways induces rapid and dramatic translational reprogramming both by in- creasing overall protein synthesis and by modulating specific mRNA networks. These trans- lational changes promote cellular transformation, impacting almost every phase of tumor development. This paradigm represents a new frontier in the multihit model of cancer for- mation and offers significant promise for innovative cancer therapies. Current research, -

Allele-Specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish
Allele-specific Expression of Ribosomal Protein Genes in Interspecific Hybrid Catfish by Ailu Chen A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama August 1, 2015 Keywords: catfish, interspecific hybrids, allele-specific expression, ribosomal protein Copyright 2015 by Ailu Chen Approved by Zhanjiang Liu, Chair, Professor, School of Fisheries, Aquaculture and Aquatic Sciences Nannan Liu, Professor, Entomology and Plant Pathology Eric Peatman, Associate Professor, School of Fisheries, Aquaculture and Aquatic Sciences Aaron M. Rashotte, Associate Professor, Biological Sciences Abstract Interspecific hybridization results in a vast reservoir of allelic variations, which may potentially contribute to phenotypical enhancement in the hybrids. Whether the allelic variations are related to the downstream phenotypic differences of interspecific hybrid is still an open question. The recently developed genome-wide allele-specific approaches that harness high- throughput sequencing technology allow direct quantification of allelic variations and gene expression patterns. In this work, I investigated allele-specific expression (ASE) pattern using RNA-Seq datasets generated from interspecific catfish hybrids. The objective of the study is to determine the ASE genes and pathways in which they are involved. Specifically, my study investigated ASE-SNPs, ASE-genes, parent-of-origins of ASE allele and how ASE would possibly contribute to heterosis. My data showed that ASE was operating in the interspecific catfish system. Of the 66,251 and 177,841 SNPs identified from the datasets of the liver and gill, 5,420 (8.2%) and 13,390 (7.5%) SNPs were identified as significant ASE-SNPs, respectively. -

Injury by Mechanical Ventilation Gene Transcription and Promotion Of
Modulation of Lipopolysaccharide-Induced Gene Transcription and Promotion of Lung Injury by Mechanical Ventilation This information is current as William A. Altemeier, Gustavo Matute-Bello, Sina A. of September 29, 2021. Gharib, Robb W. Glenny, Thomas R. Martin and W. Conrad Liles J Immunol 2005; 175:3369-3376; ; doi: 10.4049/jimmunol.175.5.3369 http://www.jimmunol.org/content/175/5/3369 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2005/08/23/175.5.3369.DC1 Material http://www.jimmunol.org/ References This article cites 37 articles, 7 of which you can access for free at: http://www.jimmunol.org/content/175/5/3369.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 29, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Modulation of Lipopolysaccharide-Induced Gene Transcription and Promotion of Lung Injury by Mechanical Ventilation1 William A. -

Structural Characterization of the Human Eukaryotic Initiation Factor 3 Protein Complex by Mass Spectrometry*□S
Supplemental Material can be found at: http://www.mcponline.org/cgi/content/full/M600399-MCP200 /DC1 Research Structural Characterization of the Human Eukaryotic Initiation Factor 3 Protein Complex by Mass Spectrometry*□S Eugen Damoc‡, Christopher S. Fraser§, Min Zhou¶, Hortense Videler¶, Greg L. Mayeurʈ, John W. B. Hersheyʈ, Jennifer A. Doudna§, Carol V. Robinson¶**, and Julie A. Leary‡ ‡‡ Protein synthesis in mammalian cells requires initiation The initiation phase of eukaryotic protein synthesis involves factor eIF3, an ϳ800-kDa protein complex that plays a formation of an 80 S ribosomal complex containing the initi- Downloaded from central role in binding of initiator methionyl-tRNA and ator methionyl-tRNAi bound to the initiation codon in the mRNA to the 40 S ribosomal subunit to form the 48 S mRNA. This is a multistep process promoted by proteins initiation complex. The eIF3 complex also prevents pre- called eukaryotic initiation factors (eIFs).1 Currently at least 12 mature association of the 40 and 60 S ribosomal subunits eIFs, composed of at least 29 distinct subunits, have been and interacts with other initiation factors involved in start identified (1). Mammalian eIF3, the largest initiation factor, is a codon selection. The molecular mechanisms by which multisubunit complex with an apparent molecular mass of www.mcponline.org eIF3 exerts these functions are poorly understood. Since ϳ800 kDa. This protein complex plays an essential role in its initial characterization in the 1970s, the exact size, translation by binding directly to the 40 S ribosomal subunit composition, and post-translational modifications of and promoting formation of the 43 S preinitiation complex ⅐ ⅐ mammalian eIF3 have not been rigorously determined. -

Blimp1 Regulates the Transition of Neonatal to Adult Intestinal Epithelium
UCLA UCLA Previously Published Works Title Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Permalink https://escholarship.org/uc/item/01x184nd Journal Nature communications, 2(1) ISSN 2041-1723 Authors Muncan, Vanesa Heijmans, Jarom Krasinski, Stephen D et al. Publication Date 2011-08-30 DOI 10.1038/ncomms1463 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ARTICLE Received 11 May 2011 | Accepted 28 Jul 2011 | Published 30 Aug 2011 DOI: 10.1038/ncomms1463 Blimp1 regulates the transition of neonatal to adult intestinal epithelium Vanesa Muncan1,2,3, Jarom Heijmans1,2,3, Stephen D. Krasinski4, Nikè V. Büller1,2,3, Manon E. Wildenberg1,2,3, Sander Meisner1, Marijana Radonjic5, Kelly A. Stapleton4, Wout H. Lamers1, Izak Biemond3, Marius A. van den Bergh Weerman6, Dónal O’Carroll7, James C. Hardwick3, Daniel W. Hommes3 & Gijs R. van den Brink1,2,3 In many mammalian species, the intestinal epithelium undergoes major changes that allow a dietary transition from mother’s milk to the adult diet at the end of the suckling period. These complex developmental changes are the result of a genetic programme intrinsic to the gut tube, but its regulators have not been identified. Here we show that transcriptional repressor B lymphocyte-induced maturation protein 1 (Blimp1) is highly expressed in the developing and postnatal intestinal epithelium until the suckling to weaning transition. Intestine-specific deletion of Blimp1 results in growth retardation and excessive neonatal mortality. Mutant mice lack all of the typical epithelial features of the suckling period and are born with features of an adult-like intestine. -

Mutations in EDA and EDAR Genes in a Large Mexican Hispanic Cohort with Hypohidrotic Ectodermal Dysplasia
Letter to the Editor http://dx.doi.org/10.5021/ad.2015.27.4.474 Mutations in EDA and EDAR Genes in a Large Mexican Hispanic Cohort with Hypohidrotic Ectodermal Dysplasia Julio C Salas-Alanis1,2, Eva Wozniak3, Charles A Mein3, Carola C Duran Mckinster4, Jorge Ocampo-Candiani1, David P Kelsell5, Rong Hua6, Maria L Garza-Rodriguez7, Keith A Choate6, Hugo A Barrera Saldaña7,8 1Dermatology Department, Hospital Universitario, Universidad Autonoma de Nuevo Leon, 2Basic Science Department, Medicine School, Universidad de Monterrey, Monterrey, NL, Mexico, 3Barts and the London Genome Centre, John Vane Science Centre, Barts and the London School of Medicine and Dentistry, University of London, London, United Kingdom, 4Instituto Nacional de Pediatria, Coyoacan, CP, Mexico, 5Centre for Cutaneous Research, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom, 6Departments of Dermatology, Genetics, and Pathology, Yale University School of Medicine, New Haven, CT, USA, 7Facultad de Medicina, Departamento de Bioquímica y Medicina Molecular, Universidad Autonoma de Nuevo Leon,8Vitagenesis, Monterrey, NL, Mexico Dear Editor: cludes dryness of the skin, eyes, airways, and mucous Ectodermal dysplasias (ED) encompass nearly 200 differ- membranes, as well as other ectodermal defects and, in ent genetic conditions identified by the lack, or dysgenesis, some cases, fever, seizures, and rarely, death. of at least two ectodermal derivatives, such as hair, nails, XL-HED is caused by mutations in the EDA gene, located teeth, and sweat glands. Hypohidrotic/anhidrotic ED (HED) on chromosome Xq12-q13.1, which encodes a signaling is the most frequent form of ED and it can be inherited as molecule of the tumor necrosis factor (TNF) superfamily. -

The Autoimmune Signature of Hyperinflammatory Multisystem Inflammatory Syndrome in Children Rebecca A. Porritt1,*, Aleksandra Bi
The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children Rebecca A. Porritt1,*, Aleksandra Binek2,*, Lisa Paschold3,*, Magali Noval Rivas1, Angela Mc Ardle2, Lael M. Yonker4,5, Galit Alter4,5,6, Harsha Chandnani7, Merrick Lopez7, Alessio Fasano4,5, Jennifer E. Van Eyk2,8,†, Mascha Binder3,† and Moshe Arditi1,9,† 1Departments of Pediatrics, Division of Infectious Diseases and Immunology, and Infectious and Immunologic Diseases Research Center (IIDRC), Department of Biomedical Sciences, Cedars- Sinai Medical Center, Los Angeles, CA, USA. 2Advanced Clinical Biosystems Research Institute, The Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA. 3Department of Internal Medicine IV, Oncology/Hematology, Martin-Luther-University Halle- Wittenberg, 06120 Halle (Saale), Germany 4Massachusetts General Hospital, Mucosal Immunology and Biology Research Center and Department of Pediatrics, Boston, MA, USA. 5Harvard Medical School, Boston, MA, USA. 6Ragon Institute of MIT, MGH and Harvard, Cambridge, MA, USA. 7Department of Pediatrics, Loma Linda University Hospital, CA, USA. 8Barbra Streisand Women's Heart Center, Cedars-Sinai Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA. 9Cedars-Sinai Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA * these authors contributed equally † these senior authors contributed equally Corresponding author: Moshe Arditi, MD 8700 Beverly Blvd. Davis Building, Rooms D4024, 4025, 4027 Los Angeles, CA 90048 Phone:310-423-4471 -

Transcriptomic Analysis of the Aquaporin (AQP) Gene Family
Pancreatology 19 (2019) 436e442 Contents lists available at ScienceDirect Pancreatology journal homepage: www.elsevier.com/locate/pan Transcriptomic analysis of the Aquaporin (AQP) gene family interactome identifies a molecular panel of four prognostic markers in patients with pancreatic ductal adenocarcinoma Dimitrios E. Magouliotis a, b, Vasiliki S. Tasiopoulou c, Konstantinos Dimas d, * Nikos Sakellaridis d, Konstantina A. Svokos e, Alexis A. Svokos f, Dimitris Zacharoulis b, a Division of Surgery and Interventional Science, Faculty of Medical Sciences, UCL, London, UK b Department of Surgery, University of Thessaly, Biopolis, Larissa, Greece c Faculty of Medicine, School of Health Sciences, University of Thessaly, Biopolis, Larissa, Greece d Department of Pharmacology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Biopolis, Larissa, Greece e The Warren Alpert Medical School of Brown University, Providence, RI, USA f Riverside Regional Medical Center, Newport News, VA, USA article info abstract Article history: Background: This study aimed to assess the differential gene expression of aquaporin (AQP) gene family Received 14 October 2018 interactome in pancreatic ductal adenocarcinoma (PDAC) using data mining techniques to identify novel Received in revised form candidate genes intervening in the pathogenicity of PDAC. 29 January 2019 Method: Transcriptome data mining techniques were used in order to construct the interactome of the Accepted 9 February 2019 AQP gene family and to determine which genes members are differentially expressed in PDAC as Available online 11 February 2019 compared to controls. The same techniques were used in order to evaluate the potential prognostic role of the differentially expressed genes. Keywords: PDAC Results: Transcriptome microarray data of four GEO datasets were incorporated, including 142 primary Aquaporin tumor samples and 104 normal pancreatic tissue samples. -

Arcn1 (NM 145985) Mouse Recombinant Protein Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TP508216 Arcn1 (NM_145985) Mouse Recombinant Protein Product data: Product Type: Recombinant Proteins Description: Purified recombinant protein of Mouse archain 1 (Arcn1), with C-terminal MYC/DDK tag, expressed in HEK293T cells, 20ug Species: Mouse Expression Host: HEK293T Tag: C-MYC/DDK Predicted MW: 57.7 kDa Concentration: >50 ug/mL as determined by microplate BCA method Purity: > 80% as determined by SDS-PAGE and Coomassie blue staining Buffer: 25 mM Tris.HCl, pH 7.3, 100 mM glycine, 10% glycerol. Storage: Store at -80°C after receiving vials. Stability: Stable for 12 months from the date of receipt of the product under proper storage and handling conditions. Avoid repeated freeze-thaw cycles. RefSeq: NP_666097 Locus ID: 213827 UniProt ID: Q5XJY5 RefSeq Size: 3470 Cytogenetics: 9 A5.2 RefSeq ORF: 1536 Synonyms: 4632432M07Rik; nur17 This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 Arcn1 (NM_145985) Mouse Recombinant Protein – TP508216 Summary: The coatomer is a cytosolic protein complex that binds to dilysine motifs and reversibly associates with Golgi non-clathrin-coated vesicles, which further mediate biosynthetic protein transport from the ER, via the Golgi up to the trans Golgi network. Coatomer complex is required for budding from Golgi membranes, and is essential for the retrograde Golgi-to-ER transport of dilysine-tagged proteins. -
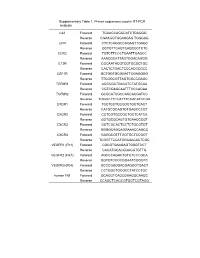
Supplementary Table 1. Primer Sequences Used in RT-PCR Analysis
Supplementary Table 1. Primer sequences used in RT-PCR analysis L32 Forward TGAAGCAGGCATCTGAGGG Reverse CGAAGGTGGAAGAG TGGGAG LIFR Forward CTCTCAGGCCAGAGTTGAGC Reverse GCTGTTCAGTCAGCCCTCTC CCR2 Forward TGTCTTCCCTGAATTGAGCC Reverse AAACGCATTAGTGGACAGGG IL12R Forward CGCAATACGTCGTGCGCTGC Reverse CACTCTGACTCCCACGCGCC CSF1R Forward GCTGGTGCGGATTCGAGGGG Reverse TTCGGCGTTAGTGGCCGAGC TGFbR1 Forward ACGCGCTGACATCTATGCAA Reverse CGTCGAGCAATTTCCCAGAA TGFbR2 Forward GCGCATCGCCAGCACGATCC Reverse TGGGCTTCCATTTCCACATCCGA CXCR1 Forward TCCTCCTGCCGCTGCTCACT Reverse CATGCGCAGTGTGAGCCCGT CXCR2 Forward CCTCGTGCCGCTGCTCATCA Reverse GGTGCGCAGTGTGAACCCGT CXCR3 Forward GGTCGCACTGCTCTGCGTGT Reverse GGGGCAGCAGGAAACCAGCC CXCR4 Forward GAGGCGTTTGGTGCTCCGGT Reverse TCGGTTCCATGGCAACACTCGC VEGFR1 (Flt1) Forward CGCGTGAAGAGTGGGTCCT Reverse CACATGCACGGAGGTGTTG VEGFR2 (Flk1) Forward AGCCCAGACTGTGTCCCGCA Reverse GGTGTCCGCGGAATCGGGTC VEGFR3 (Flt4) Forward GCCCGAGGACGAGGGTGACT Reverse CCTGGCTGCGCCTATCCTGC human Flt1 Forward GCACGTCAGCGAAGGCAAGC Reverse CCAGCTCAGCGTGGTCGTAGG Supplementary Table 2. List of putative miR-200 target genes amplified in human cancers amplified in cancers (Tumorscape)a Gene Symbol Title various cancers NSCLC PCT Errfi1 ERBB receptor feedback inhibitor 1 - yes 0.94 D11Bwg0517e DNA segment, Chr 11, Brigham & Women's Genetics 0517 expressed - yes 0.91 Pvrl4 poliovirus receptor-related 4 yes - 0.91 Mecp2 methyl CpG binding protein 2 yes yes 0.9 Jun Jun oncogene yes - 0.88 Prdm16 PR domain containing 16 - yes 0.88 Flt1 FMS-like tyrosine kinase 1 yes -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Yeast Genome Gazetteer P35-65
gazetteer Metabolism 35 tRNA modification mitochondrial transport amino-acid metabolism other tRNA-transcription activities vesicular transport (Golgi network, etc.) nitrogen and sulphur metabolism mRNA synthesis peroxisomal transport nucleotide metabolism mRNA processing (splicing) vacuolar transport phosphate metabolism mRNA processing (5’-end, 3’-end processing extracellular transport carbohydrate metabolism and mRNA degradation) cellular import lipid, fatty-acid and sterol metabolism other mRNA-transcription activities other intracellular-transport activities biosynthesis of vitamins, cofactors and RNA transport prosthetic groups other transcription activities Cellular organization and biogenesis 54 ionic homeostasis organization and biogenesis of cell wall and Protein synthesis 48 plasma membrane Energy 40 ribosomal proteins organization and biogenesis of glycolysis translation (initiation,elongation and cytoskeleton gluconeogenesis termination) organization and biogenesis of endoplasmic pentose-phosphate pathway translational control reticulum and Golgi tricarboxylic-acid pathway tRNA synthetases organization and biogenesis of chromosome respiration other protein-synthesis activities structure fermentation mitochondrial organization and biogenesis metabolism of energy reserves (glycogen Protein destination 49 peroxisomal organization and biogenesis and trehalose) protein folding and stabilization endosomal organization and biogenesis other energy-generation activities protein targeting, sorting and translocation vacuolar and lysosomal