Revision of the Leucosphyrus Group of Anopheles (Cellia) (Diptera, Culicidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Myanmar: the Key Link Between
ADBI Working Paper Series Myanmar: The Key Link between South Asia and Southeast Asia Hector Florento and Maria Isabela Corpuz No. 506 December 2014 Asian Development Bank Institute Hector Florento and Maria Isabela Corpuz are consultants at the Office of Regional Economic Integration, Asian Development Bank. The views expressed in this paper are the views of the author and do not necessarily reflect the views or policies of ADBI, ADB, its Board of Directors, or the governments they represent. ADBI does not guarantee the accuracy of the data included in this paper and accepts no responsibility for any consequences of their use. Terminology used may not necessarily be consistent with ADB official terms. Working papers are subject to formal revision and correction before they are finalized and considered published. In this paper, “$” refers to US dollars. The Working Paper series is a continuation of the formerly named Discussion Paper series; the numbering of the papers continued without interruption or change. ADBI’s working papers reflect initial ideas on a topic and are posted online for discussion. ADBI encourages readers to post their comments on the main page for each working paper (given in the citation below). Some working papers may develop into other forms of publication. Suggested citation: Florento, H., and M. I. Corpuz. 2014. Myanmar: The Key Link between South Asia and Southeast Asia. ADBI Working Paper 506. Tokyo: Asian Development Bank Institute. Available: http://www.adbi.org/working- paper/2014/12/12/6517.myanmar.key.link.south.southeast.asia/ Please contact the authors for information about this paper. -

BURMA/THAILAND No Safety in Burma, No Sanctuary in Thailand
July 1997 Vol. 9, No. 6 (C) BURMA/THAILAND No Safety in Burma, No Sanctuary in Thailand I. SUMMARY AND RECOMMENDATIONS .........................................................................................................2 Recommendations..........................................................................................................................................3 II. BACKGROUND ...................................................................................................................................................6 III. REFUGEES FROM BURMA'S KAREN AND MON STATES AND TENASSERIM DIVISION ..................7 Human Rights Violations by the Burmese Military.......................................................................................7 Repatriations and Denial of Access By the Royal Thai Government ..........................................................13 Instances of Refoulement.............................................................................................................................13 Attacks on the Refugee Camps ....................................................................................................................18 Conditions in the Refugee Camps................................................................................................................19 IV. SITUATION OF THOSE FROM BURMA'S SHAN STATE...........................................................................20 Human Rights Violations by the Burmese Military.....................................................................................20 -

Intermodal Logisticslogistics Unlockingunlocking Vvaluealue
IntermodalIntermodal LogisticsLogistics UnlockingUnlocking VValuealue Asian Institute of Transport Development E-5, Qutab Hotel, Shaheed Jeet Singh Marg, New Delhi 110 016, India Tel: (91-11) 26856117 Telefax: (91-11) 26856113 Email: [email protected] ASIAN INSTITUTE OF TRANSPORT DEVELOPMENT Intermodal Logistics: Unlocking Value © Asian Institute of Transport Development, New Delhi First published 2007 All rights reserved Published by Asian Institute of Transport Development E-5, Qutab Hotel Shaheed Jeet Singh Marg New Delhi-110 016 INDIA Phones: +91-11-26856117, 26856113 Fax: +91-11-26856113 Email: [email protected], [email protected] Intermodal Logistics Unlocking Value ASIAN INSTITUTE OF TRANSPORT DEVELOPMENT CONTENTS Preface i Abbreviations iii Contextual Information vii Chapter 1: Globalisation and Logistics: The Container Revolution 1 Section I : Maritime Transport Logistics Chapter 2: Maritime Transport Networks and Container Shipping Development 27 Chapter 3: A New Ports Structure: Asia Moves Ahead 45 Section II: Land Transport Logistics Chapter 4: Land Transport Networks: Regional and Subregional 85 Chapter 5: Dry Ports: Sharing Benefits 153 Section III: Facilitation of Multimodal Transport Logistics Chapter 6: Institutional Framework: Cross-border Impediments 167 Section IV: The Way Ahead 193 Annexures 199 References 205 Preface For about 25 years now, national barriers to trade and investment have been dismantled at an unprecedented pace, leading to a degree of integration of product and financial markets that is reminiscent of the pre- twentieth century global economic arrangements. One of the consequences of this has been the spread of production facilities across national borders. Thus, what was started by US multinational firms in the 1960s in Europe has now become a global trend. -

Highway Network Project
MINISTERIAL UNDERSTANDING DEVELOPMENE ONTH F TO THE ASEAN HIGHWAY NETWORK PROJECT WE the undersigned, attending the Fifth ASEAN Transport Ministers Meeting held in Hanoi, Vietnam on 15-16 September 1999; RECALLING the following policy directives enunciated by the ASEAN Heads of Stat Governmentd ean : 1. Manila Declaratio f 198no 7 Decembesigne5 1 n do r 1987, which provided that the existing transportation system shall be strengthened to ultimately form an overall ASEAN transportation network; 2. ASEAN Vision 2020 adopted in Kuala Lumpur, Malaysia on 15 December 1997, which resolved, inter alia, the development of an integrated and harmonized trans-ASEAN transportation network; 3. Hanoi Plan of Action adopted on 15 December 1998, which provided for intensifying cooperation in the development of the trans-ASEAN transportation network as the trunkline or main corridor for the movement of goods and people in ASEAN, consistin f majoo g r road (interstate highway) networks, among e developmenth others d an , d implementatioan t e ASEAth f o nN Highway Network Project; and 4. Hanoi Declaratio f 199no 8 signeDecembe6 1 n do r 1998, which e calleth r dfo development and strengthening of ASEAN regional infrastructure through the expansion of transport links and the provision of efficient and quality infrastructure. RECALLING FURTHE r resolvRou e unde e Ministeriath r l Understandinn o g ASEAN Cooperatio Transportation ni n signe Balin di , Indonesi Marc8 1 n aho 1996, to establis develod han pharmonizea integrated dan d regional transportation -
R-PIN Template
FCPF R-PIN Template TheForest Forest Carbon Carbon Partnership Partnership Facility Facility (FCPF) (FCPF ) ReadinessReadiness Plan Plan Idea Idea Note Note (R (R-PIN)-PIN) Template Template R-PIN Format Version of March 8, 2008 February 20, 2008 Guidelines: 1. The purpose of this document is to: a) request an overview of your country’s interest in the FCPF program, and b) provide an overview of land use patterns, causes of deforestation, stakeholder consultation process, and potential institutional arrangements in addressing REDD (Reducing Emissions from Deforestation and Forest degradation). This R-PIN will be used as a basis for the selection of countries into the FCPF by the Participants Committee. Information about the FCPF is available at: www.carbonfinance.org/fcpf 2. Please keep the length of your response under 20 pages. You may consider using the optional Annex 1 Questionnaire (at the end of this template) to help organize some answers or provide other information. 3. You may also attach at most 15 additional pages of technical material (e.g., maps, data tables, etc.), but this is optional. If additional information is required, the FCPF will request it. 4. The text can be prepared in Word or other software and then pasted into this format. 5. For the purpose of this template, “Deforestation” is defined as the change in land cover status from forest to non- forest (i.e., when harvest or the gradual degrading of forest land reduces tree cover per hectare below your country’s definition of “forest.” “Forest degradation” is the reduction of tree cover and forest biomass per hectare, via selective harvest, fuel wood cutting or other practices, but where the land still meets your country’s definition of “forest” land. -

CSO and Community Contribution to Malaria Elimination in Thailand
CSO and Community Contribution to Malaria Elimination in Thailand October 2019 1 | P a g e Report prepared by Alistair Shaw Raks Thai Foundation Based on consultation with, and contribution from Raks Thai Foundation, American Refugee Committee, Pattanarak Foundation, World Vision Foundation of Thailand, Young Muslim Association of Thailand, Stella Maris Seafarer’s Center, the International Organization for Migration and Shoklo Malaria Research Unit With financial and logistics support from The Regional CSO Malaria Platform, hosted by the American Refugee Committee, and Raks Thai Foundation 2 | P a g e Table of Contents Executive Summary................................................................................................ 4 Civil Society Organizations in Thailand ................................................................... 5 Role and Added-Value ....................................................................................................................5 Raks Thai Foundation .....................................................................................................................6 American Refugee Committee ........................................................................................................7 Pattanarak Foundation ...................................................................................................................8 World Vision Foundation of Thailand ..............................................................................................9 Young Muslim Association -

Connecting Asia
Connecting Asia ADBI SERIES ON ASIAN ECONOMIC INTEGRATION AND COOPERATION Previous titles published in association with the ADBI include: Infrastructure and Trade in Asia Edited by Douglas H. Brooks and Jayant Menon Infrastructure’s Role in Lowering Asia’s Trade Costs Building for Trade Edited by Douglas H. Brooks and David Hummels Trade Facilitation and Regional Cooperation in Asia Edited by Douglas H. Brooks and Susan F. Stone Managing Capital Flows The Search for a Framework Edited by Masahiro Kawai and Mario B. Lamberte The Asian Tsunami Aid and Reconstruction after a Disaster Edited by Sisira Jayasuriya and Peter McCawley Asia’s Free Trade Agreements How is Business Responding? Edited by Masahiro Kawai and Ganeshan Wignaraja Monetary and Currency Policy Management in Asia Edited by Masahiro Kawai, Peter J. Morgan and Shinji Takagi Implications of the Global Financial Crisis for Financial Reform and Regulation in Asia Edited by Masahiro Kawai, David G. Mayes and Peter J. Morgan Infrastructure for Asian Connectivity Edited by Biswa Nath Bhattacharyay, Masahiro Kawai and Rajat M. Nag A World Trade Organization for the 21st Century The Asian Perspective Edited by Richard Baldwin, Masahiro Kawai and Ganeshan Wignaraja New Global Economic Architecture The Asian Perspective Edited by Masahiro Kawai, Peter J. Morgan and Pradumna B. Rana Connecting Asia Infrastructure for Integrating South and Southeast Asia Edited by Michael G. Plummer, Peter J. Morgan and Ganeshan Wignaraja The Asian Development Bank Institute (ADBI), located in Tokyo, is the think tank of the Asian Development Bank (ADB). ADBI’s mission is to identify effec- tive development strategies and improve development management in ADB’s developing member countries. -
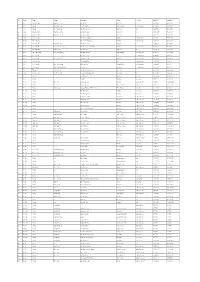
List Station.Pdf
No. Code River Stream At or Near District Province Lat_Edit Long_Edit 1 Sw.1 Nam Mae Moei Huai Mae Lamao Ban Ko Ko Mae Sot Tak 16.919312 98.631453 2 Sw.2 Salawin Mae Nam Yuam Ban Tha Kham Mae Sariang Mae Hong Son 18.191790 97.931669 3 Sw.3 Salawin Nam Mae Moei Ban Tha Sai Luat Mae Sot Tak 16.687951 98.515674 4 Sw.4 Nam Mae Moei Huai Mae Lamao Ban Mae Lamao Mae Sot Tak 16.806828 98.743108 5 Sw.4A Nam Mae Moei Huai Mae Lamao Ban Mae Lamao Mae Sot Tak 16.805149 98.742724 6 Sw.5 Mae Nam Pai - Ban Tha Pong Daeng Mueang Mae Hong Son 19.269749 97.944970 7 Sw.5A Mae Nam Pai - Ban Tha Pong Daeng Mueang Mae Hong Son 19.270330 97.945209 8 Sw.6 Nam Mae Moei Huai Mae Lamao Ban Mae Lamao Mae Sot Tak 16.764025 98.751540 9 Sw.7 Mae Nam Pai Nam Mae Hong Son Ban Mai Hua, Sanam Bin Mueang Mae Hong Son 19.289643 97.991043 10 Sw.8 Nam Mae Moei Huai Pha Wo Ban Pha Wo Mae Sot Tak 16.809207 98.733936 11 Sw.9 Mae Nam Yuam Nam Mae Sariang Ban Mae Sariang Mae Sariang Mae Hong Son 18.164047 97.954886 12 Sw.10 Mae Nam Pai - Ban Sop Phaem Pai Mae Hong Son 19.249658 98.453301 13 Sw.11 Mae Nam Pai Nam Mae Sanga Ban Mae Sanga Mueang Mae Hong Son 19.405928 97.952620 14 Sw.12 Mae Nam Pai - Ban Wiang Tai Pai Mae Hong Son 19.355000 98.444391 15 Sw.13 Mae Nam Pai Nam Mae Khong Ban Huai San Pang Ma Pha Mae Hong Son 19.534979 98.116071 16 Sw.14 Mae Nam Pai Huai Nam Pang Hung Ban Nam Kat Mueang Mae Hong Son 19.505374 98.075157 17 Sw.15 Salawin Huai Mae Sot Ban Khun Huai Mae Sot Mae Sot Tak 16.711447 98.665896 18 Sw.16 Huai Mae Sot Ban Thung Noi Ban Khun Huai Mae Sot Mae Sot Tak -

Very High Carriage of Gametocytes in Asymptomatic Low-Density Plasmodium Falciparum and P
Nguitragool et al. Parasites & Vectors (2017) 10:512 DOI 10.1186/s13071-017-2407-y RESEARCH Open Access Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand Wang Nguitragool1†, Ivo Mueller2,3,4†, Chalermpon Kumpitak5, Teerawat Saeseu5, Sirasate Bantuchai5, Ritthideach Yorsaeng5, Surapon Yimsamran6, Wanchai Maneeboonyang6, Patiwat Sa-angchai6, Wutthichai Chaimungkun6, Prasert Rukmanee6, Supalarp Puangsa-art6, Nipon Thanyavanich6, Cristian Koepfli3,4, Ingrid Felger7, Jetsumon Sattabongkot5 and Pratap Singhasivanon6* Abstract Background: Low-density asymptomatic infections of Plasmodium spp. are common in low endemicity areas worldwide, but outside Africa, their contribution to malaria transmission is poorly understood. Community-based studies with highly sensitive molecular diagnostics are needed to quantify the asymptomatic reservoir of Plasmodium falciparum and P. vivax infections in Thai communities. Methods: A cross-sectional survey of 4309 participants was conducted in three endemic areas in Kanchanaburi and Ratchaburi provinces of Thailand in 2012. The presence of P. falciparum and P. vivax parasites was determined using 18S rRNA qPCR. Gametocytes were also detected by pfs25 / pvs25 qRT-PCRs. Results: A total of 133 individuals were found infected with P. vivax (3.09%), 37 with P. falciparum (0.86%), and 11 with mixed P. vivax/ P. falciparum (0.26%). The clear majority of both P. vivax (91.7%) and P. falciparum (89.8%) infections were not accompanied by any febrile symptoms. Infections with either species were most common in adolescent and adult males. Recent travel to Myanmar was highly associated with P. falciparum (OR = 9.0, P = 0.001) but not P. vivax infections (P = 0.13). -

Progress Report on the GMS RIF: Transport and Related Services
Progress Report on the Regional Investment Framework Following the endorsement of the new GMS Strategic Framework (SF) by heads of state at the 4th GMS Summit in Myanmar in December 2011, it was agreed that a Regional Investment Framework (RIF) be formulated to identify and develop the investment pipeline as well as the software and other aspects of GMS regional cooperation in order to ensure the effective operationalization of the new SF. Accordingly during the course of this year analytical work has been undertaken from both a country and a sector/program perspective under ADB technical assistance project funded by the Government of Australia. The RIF Sector Reports are provided to present the findings of assessments used as inputs for the formulation of the RIF. At sector level, a dual approach was adopted in assessing the forward direction for the different sectors of GMS cooperation, with a combination of new assessments and use of existing strategies. New assessments were undertaken for the transport, energy and urban development sectors, as well as for migration trends in the GMS that provided inputs into the concurrent formulation of a strategic framework and action plan for the human resource development sector. For other sectors, specifically agriculture, environment, and tourism, the formulation of the RIF has drawn on existing sector strategies that were approved recently. REGIONAL INVESTMENT FRAMEWORK SECTOR REPORT TRANSPORT AND RELATED SERVICES 18th GMS Ministerial Conference Nanning, People’s Republic of China 11–12 December 2012 Printed on recycled paper Printed in the Philippines Initial Assessments of Road Transport Infrastructure and Transport and Logistic Services for Trade Facilitation in the GMS Countries: Draft Final Report The views expressed in this working paper are those of the authors and do not necessarily reflect the views and policies of the Asian Development Bank (ADB) or its Board of Governors or the governments they represent. -

Highly Heterogeneous Residual Malaria Risk in Western Thailand
International Journal for Parasitology 49 (2019) 455–462 Contents lists available at ScienceDirect International Journal for Parasitology journal homepage: www.elsevier.com/locate/ijpara Highly heterogeneous residual malaria risk in western Thailand Wang Nguitragool a, Stephan Karl b,c,d, Michael White e, Cristian Koepfli b,c, Ingrid Felger f, ⇑ ⇑ Pratap Singhasivanon g, Ivo Mueller b,c,e, , Jetsumon Sattabongkot h, a Department of Molecular Tropical Medicine & Genetics, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand b Population Health and Immunity Division, Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia c Department of Medical Biology, University of Melbourne, Parkville, Victoria, Australia d Vector-borne Diseases Unit, Papua New Guinea Institute of Medical Research, Madang, Madang Province, Papua New Guinea e Malaria: Parasites and Hosts Unit, Department of Parasites & Insect Vectors, Institute Pasteur, Paris, France f Department of Medical Parasitology and Infection Biology, Swiss Tropical & Public Health Institute, Basel, Switzerland g Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand h Mahidol Vivax Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand article info abstract Article history: Over the past decades, the malaria burden in Thailand has substantially declined. Most infections now Received 3 August 2018 originate from the national border regions. In these areas, the prevalence of asymptomatic infections is Received in revised form 21 January 2019 still substantial and poses a challenge for the national malaria elimination program. To determine epi- Accepted 25 January 2019 demiological parameters as well as risk factors for malaria infection in western Thailand, we carried Available online 4 April 2019 out a cohort study in Kanchanaburi and Ratchaburi provinces on the Thailand-Myanmar border. -

Cross Learning Field Visit Kanchanaburi Province, Thailand 14 - 15 September 2018
Cross Learning Field Visit Kanchanaburi province, Thailand 14 - 15 September 2018 American Refugee Committee, host of the Malaria CSO platform with support from platform partner Raks Thai Foundation organized a learning visit last 14-15 September 2018 in Kanchanaburi Province, Thailand which is one of the RAI2E implementing areas along the Thailand-Myanmar border. The main objectives of the visit were to interact with community people malaria volunteers, community leaders, and malaria risk population and service providers and identify the success and challenges at the field level, and document evidence of success and challenges in RAI2E implementation and share to the RAI RSC .Meeting/discussion with project staffs, volunteers, public health officers, Village Malaria Worker (VMW), Mobile Malaria Worker (MMW) and community people were organized during the visit . This report was developed as part of a collaborative cross learning field visit activity coordinated by RAI RSC CSO platform in GMS. The activity enabled RAI RSC CSO representative and other CSOs to interact with and learn from each other, allowing them to view and share practical experience of best practice, challenges and success in malaria response. This report is based on observation in the RAI implementing districts of Kanchanaburi and cannot be generalized for other areas. ARC on behalf of Malaria CSO platform would like to thank you for the generosity of the individuals and partners for their support to this visit. About the platform The Regional Malaria CSO Platform in the Greater Mekong Sub-region (GMS) is a network of Civil Society Organizations (CSO) from the Global Fund RAI implementing countries: Myanmar, Thailand, Cambodia, Lao PDR, and Vietnam.