Sample Chapter 17 from Optometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sightsavers Policy on Recycled Spectacles
DRAFT POSITION PAPER ADJUSTABLE SPECTACLES Position statement There are an estimated 670 million cases of blindness or vision impairment (153 million people with impaired far visioni and 517 million people with impaired near visionii) simply because they are unable to access an appropriate eye examination and spectacles. Far vision impairment alone costs $269billion a year in lost productivity.iii The massive volume of cases, and consequent scale of disability and economic impact caused by uncorrected refractive error (URE) has lead to various attempts to provide short-cut refractive care. Adjustable spectacles, with the optical power either set in an eye examination or self-adjusted, have been promoted by several companies and organizations as a potential solution.iv IAPB recognises the good intentions behind these short-cuts and spectacle technology but advises that its members and other parties engaged in promoting eye health should exercise caution with or avoid adjustable spectacles at this time. In deciding whether and how to use adjustable spectacles in future, the following areas should be considered. Considerations Sustainability It is critical that refractive services are provided in a manner that contributes to affordable, high quality eye care for all patients irrespective of social standing. Isolated provision of spectacles is detrimental to the human resources and service delivery systems that are necessary for sustainable eye care services, and so should be avoided whenever possible. • Adjustable spectacles that are affordable, comfortable, cosmetically acceptable and optically accurate may have a role contributing to sustainability when used by trained personnel within the recognised service delivery system of the jurisdiction • Adjustable spectacles used in a way (e.g. -

Introduction the Human
Physics 1CL ·OPTICAL INSTRUMENTS AND THE EYE SPRING 2010 Introduction Most of the subject material in this lab can be found in Chapter 25 of Serway and Faughn. In this lab, you will make images of images using lenses and the optical bench (Experiment A). IT IS IMPERATIVE THAT YOU READ CHAPTER 25 BEFORE COMING TO LAB! You will study your own eye as an optical instrument and measure the distance on your retina from the fovea to the “blind spot” (Experiment B). You will also study a model of the eye, and examine the ability of the lens to bring an object into focus at different distances. You will examine how an abnormal eyeball causes blurred vision and how to correct this (Experiment C). There is only one station with the model of the human eye. Whenever that station becomes available, take a few minutes at that station to perform Experiment C. The Human Eye The figure shows a cross section of a human eye. Light is refracted at the surface of the cornea, and is refracted again as it passes through the “crystalline” lens. In a normal eye, light is perfectly focused on the receptors in the retina where signals are generated in nerve fibers and transmitted to the brain. An inverted image is formed on the retina, but the brain “expects” this as normal and is wired to recognize this as normal. Unless you are studying details of the function of the eye, we can consider it to be a single lens (where the focal length can be varied), and a fixed distance from the lens to the retina where we would prefer to make sharply focused images. -

Relationship Between Lenticular Power and Refractive Error in Children with Hyperopia
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Relationship between lenticular power and refractive error in children with hyperopia Takeshi Tomomatsu Objectives: To evaluate the contribution of axial length, and lenticular and corneal power to Shinjiro Kono the spherical equivalent refractive error in children with hyperopia between 3 and 13 years of Shogo Arimura age, using noncontact optical biometry. Yoko Tomomatsu Methods: There were 62 children between 3 and 13 years of age with hyperopia (+2 diopters Takehiro Matsumura [D] or more) who underwent automated refraction measurement with cycloplegia, to measure Yuji Takihara spherical equivalent refractive error and corneal power. Axial length was measured using an optic biometer that does not require contact with the cornea. The refractive power of the lens Masaru Inatani was calculated using the Sanders-Retzlaff-Kraff formula. Single regression analysis was used Yoshihiro Takamura to evaluate the correlation among the optical parameters. For personal use only. Department of Ophthalmology, Results: There was a significant positive correlation between age and axial length P( = 0.0014); Faculty of Medical Sciences, University of Fukui, Fukuiken, Japan however, the degree of hyperopia did not decrease with aging (P = 0.59). There was a significant negative correlation between age and the refractive power of the lens (P = 0.0001) but not that of the cornea (P = 0.43). A significant negative correlation was observed between the degree of hyperopia and lenticular power (P , 0.0001). Conclusion: Although this study is small scale and cross sectional, the analysis, using noncontact biometry, showed that lenticular power was negatively correlated with refractive error and age, indicating that lower lens power may contribute to the degree of hyperopia. -

I. Multiple Mechanisms of Accommodation A. Variable Axial Length B
Mechanics, Aging and Neurological Control of accommodation: I. Multiple Mechanisms of Accommodation A. Variable axial length B. Corneal Power C. Lenticular power D. Pupil size E. Lenticular refractive index gradient (isoindical surfaces) II. Anatomy A. Lens B. Capsule C. Zonules D. Ciliary Body E. Index gradient III. Autonomic innervation IV. Amplitude of accommodation and age A. Functional presbyopia B. Absolute presbyopia C. Treatment Bifocals Monovision Surgically implanted prosthesis Course title - (VS217) Oculomotor functions and neurology Instructor - Clifton Schor GSI: James O’Shea, Michael Oliver & Aleks Polosukhina Schedule of lectures, exams and laboratories : Lecture hours 10-11:30 Tu Th; 5 min break at 11:00 Labs Friday the first 3 weeks Examination Schedule : Quizes: January 29; February 28 Midterm: February 14: Final March 13 Power point lecture slides are available on a CD Resources: text books, reader , website, handouts Class Website: Reader. Website http://schorlab.berkeley.edu Click courses 117 class page name VS117 password Hering,1 First Week: read chapters 16-18 See lecture outline in syllabus Labs begin this Friday, January 25 Course Goals Near Response - Current developments in optometry Myopia control – environmental, surgical, pharmaceutical and genetic Presbyopia treatment – amelioration and prosthetic treatment Developmental disorders (amblyopia and strabismus) Reading disorders Ergonomics- computers and sports vision Virtual reality and personal computer eye-ware Neurology screening- Primary care gate keeper neurology, systemic, endocrines, metabolic, muscular skeletal systems. Mechanics, Aging and Neurological Control of accommodation : I. Five Mechanisms of Accommodation A. Variable axial length B. Corneal Power and astigmatism C. Lenticular power D. Pupil size & Aberrations E. Lenticular refractive index gradient (isoindical surfaces) II. -

6. Refractive Errors .Pdf
Refractive Errors Objectives: ● Not given. [ Color index : Important | Notes | Extra ] Resources: Slides+Notes+Lecture notes of ophthalmology+434team. Done by : Abdulrahman Al-Shammari & Munira alhussaini Edited by: Munerah alOmari Revised by : Adel Al Shihri, Lina Alshehri. FACTS: ● 75% of avoidable blindness is due to: ● Uncorrected refractive error ● Cataract ● Trachoma Physiology: ● To have a clear picture in the retina & to be seen in the brain, there should be a clear cornea, clear anterior chamber, and clear lens, clear vitreous cavity then the picture should be focused on the retina with normal refractive index. ● The retina is responsible for the perception of light. It converts light rays into impulses; sent through the optic nerve to your brain, where they are recognized as images. ● Normal refractive power of the eye is 60 diopters. (The cornea accounts for approximately two-thirds .(and the crystalline lens contributes the remaining (ﺛﺎﺑﺖ of this refractive power (about 40 diopters 60 is the power when we’re looking at something far ( the lens is relaxed). but when we look at near objects the lens’s power increases according to the distance of the object we’re looking at. ● The normal axial length is 22.5 mml (it’s measured from the tip of the cornea to the surface of the retina). ● If the axial length is longer = the picture will be in front of the retina “Myopia”. ● If the axial length is shorter = the picture will be behind the retina “Hyperopia”. ❖Refraction: ● In optics, refraction occurs when light waves travel from a medium with a given refractive index to a medium with another. -

Behavioral Optometric Science Student Manual
Pacific University CommonKnowledge College of Optometry Theses, Dissertations and Capstone Projects 9-2004 Behavioral optometric science student manual Kristel Rogers Pacific University Recommended Citation Rogers, Kristel, "Behavioral optometric science student manual" (2004). College of Optometry. 1486. https://commons.pacificu.edu/opt/1486 This Thesis is brought to you for free and open access by the Theses, Dissertations and Capstone Projects at CommonKnowledge. It has been accepted for inclusion in College of Optometry by an authorized administrator of CommonKnowledge. For more information, please contact [email protected]. Behavioral optometric science student manual Abstract Purpose: To provide the first year optometry student with a simplified guide to behavioral optometry terminology and concepts. The goal is for the student to gain a better understanding of behavioral optometry throughout the first semester. Methods: The coursework from Optometry 562 has been broken into four sections. Each author has written about one of these sections in her own perspective as another way to explain behavioral optometry. This publication is one of the four sections written. It covers types of refractive conditions, their etiology, and how to compensate for them. It also includes affects of stress on vision and theories on adaptation to visual stress both short-term and long-term. Lastly, classification and etiology of myopia is discussed to better understand this common refractive condition. Other Authors of Manual: Angela Darveaux, Katherine Chhor, Kim Schweiger Degree Type Thesis Degree Name Master of Science in Vision Science Committee Chair Bradley Coffey Subject Categories Optometry This thesis is available at CommonKnowledge: https://commons.pacificu.edu/opt/1486 Copyright and terms of use If you have downloaded this document directly from the web or from CommonKnowledge, see the “Rights” section on the previous page for the terms of use. -

Modeling of Mouse Eye and Errors in Ocular Parameters Affecting Refractive State Gurinder Bawa Wayne State University
Wayne State University Wayne State University Dissertations 1-1-2013 Modeling Of Mouse Eye And Errors In Ocular Parameters Affecting Refractive State Gurinder Bawa Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Part of the Electrical and Computer Engineering Commons, and the Optics Commons Recommended Citation Bawa, Gurinder, "Modeling Of Mouse Eye And Errors In Ocular Parameters Affecting Refractive State" (2013). Wayne State University Dissertations. Paper 749. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. MODELING OF MOUSE EYE AND ERRORS IN OCULAR PARAMETERS AFFECTING REFRACTIVE STATE by GURINDER BAWA DISSERTATION Submitted to the Graduate School of Wayne State University Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2013 MAJOR: ELECTRICAL ENGINEERING Approved by: Advisor Date © COPYRIGHT BY GURINDER BAWA 2013 All Rights Reserved DEDICATION Dedicated to My father Jagjit Singh Bawa, mother Swinderjit Kaur and dearest wife Simerjeet Kaur for their endless support and Love & To my late grandfather Gurbaksh Singh Bhalla and late grandmother Agya Wanti. ii ACKNOWLEDGEMENTS First and foremost, above all I would like to express my sincere gratitude to my principal advisor Dr. Ivan Avrutsky, who always stood by me during the time of need, while advising me. I am really thankful to him for extending his valuable, time, guidance and advice. I am grateful to all my committee members Dr. Andrei Tkatchenko, Dr. Mark Cheng and Dr. -

Vertex Distance, Effective Power, and Compensated Power – Tilt – Wrap
Continuing Education Course Approved by the American Board of Opticianry Vertex Distance, Effective Power, and Compensated Power – Tilt – Wrap National Academy of Opticianry 8401 Corporate Drive #605 Landover, MD 20785 800-229-4828 phone 301-577-3880 fax www.nao.org Copyright© 2016 by the National Academy of Opticianry. All rights reserved. No part of this text may be reproduced without permission in writing from the publisher. 2 National Academy of Opticianry PREFACE: This continuing education course was prepared under the auspices of the National Academy of Opticianry and is designed to be convenient, cost effective and practical for the Optician. The skills and knowledge required to practice the profession of Opticianry will continue to change in the future as advances in technology are applied to the eye care specialty. Higher rates of obsolescence will result in an increased tempo of change as well as knowledge to meet these changes. The National Academy of Opticianry recognizes the need to provide a Continuing Education Program for all Opticians. This course has been developed as a part of the overall program to enable Opticians to develop and improve their technical knowledge and skills in their chosen profession. The National Academy of Opticianry INSTRUCTIONS: Read and study the material. After you feel that you understand the material thoroughly take the test following the instructions given at the beginning of the test. Upon completion of the test, mail the answer sheet to the National Academy of Opticianry, 8401 Corporate Drive, Suite 605, Landover, Maryland 20785 or fax it to 301-577-3880. Be sure you complete the evaluation form on the answer sheet. -

Insight Into Why Some Children Develop Myopia Insight Into Why Some Children Develop Myopia
Insight into why some children develop myopia Insight into why some children develop myopia March 2, 2012 at 4:15 AM Myopia (nearsightedness) develops in children when the lens stops compensating for continued growth of the eye, according to a study in the March issue of Optometry and Vision Science, official journal of the American Academy of Optometry. The journal is published by Lippincott Williams & Wilkins, a part of Wolters Kluwer Health. Using detailed information on eye growth and vision changes in children over time, the new research shows "decoupling" of lens adaptation from eye growth about a year before myopia occurs. Donald O. Mutti, OD, PhD, of The Ohio State University College of Optometry, is lead author of the new study. Growth Imbalance Leads to Myopia… The researchers analyzed repeated measurements of vision and eye growth performed over several years in children aged 6 to 14. The study focused on the growth of the two key parts of the eye affecting normal vision: the cornea, the transparent front part that lets light into the eye; and the lens, located behind the cornea, which focuses light rays on the retina at the back of the eye. Myopia or nearsightedness—difficulty seeing objects at a distance—develops in about 34% of American children as they grow. Vision professionals and scientists typically think of myopia as a problem occurring when the eyeball becomes too long (front to back) for the optical power of the cornea and lens. However, it has been unclear how this imbalance develops in children who previously had normal vision. -

LENSOMETRY Handout Sandra Fortenberry, O.D
LENSOMETRY Handout Sandra Fortenberry, O.D. Tami Hagemeyer, A.B.O.C. TERMINOLOGY Lensometer – instrument designed to measure the prescription of an optical lens. Mires – lines, thick and thin used as measurement images. Power Drum or Power Wheel –dial used to determine lens power. Platform‐ stage the frame rests on when lenses are being neutralized Instrument consists of an ocular for viewing the mires, a flat stage or table for supporting the spectacle frame, a power dial, and an axis wheel. Sphere – lens with optical power being the same in all meridians (conveyed in diopters). Cylinder – lens that has different refractive/optical power in each meridian. It is used to correct astigmatism. Axis – meridian of cylinder with the minimum power perpendicular to maximum power meridian. Expressed in degrees. Prism ‐ a transparent, wedge shaped material with two flat surfaces inclined at a given angle that connect at a point called the apex. The two connected surfaces are resting on the base of the prism. Prisms are used to help the eyes to work together by bending or refracting light. Lensometer Functions The function of a Lensometer is to determine the characteristics of a lens, including: 1. Power 2. Optical Center location 3. Major Reference Point location 4. Prism power/direction 5. Cylinder axis orientation The Lensometer is also used to place marks on a lens to ensure proper placement of the lens during the fabrication process. Lensometry Measurement: Procedure for Single Vision Lenses a. Set power wheel to zero b. Set the prism compensator to zero c. Focus the eyepiece 1. -
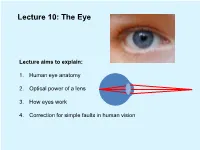
Lecture Aims to Explain: 1. Human Eye Anatomy 2. Optical Power of a Lens
Lecture 10: The Eye Lecture aims to explain: 1. Human eye anatomy 2. Optical power of a lens 3. How eyes work 4. Correction for simple faults in human vision Human eye anatomy Human eye anatomy Light passes through transparent tissue cornea first element in the eye's focusing system Then iris controls the amount of light entering the eye The adjustable crystalline lens – a biconvex body helping to focus the light on retina a layer of light-sensitive tissue at the back of the eye. The retina contains light-sensitive cells called photoreceptors, which translate the light energy into electrical signals fed into optic nerve for delivery to the brain. Photoreceptors in the retina: cones and rods Rods ~120 million on the retina, fast and sensitive “black & white” light detectors, responsible for vision in reduced light levels Cones ~6 million on the retina, slow and less sensitive “colour sensitive” light detectors Rods ~ 100 – 1000 times more sensitive to light than cones Optical power of a lens Optical power of a lens Each surface bends the incoming rays: the more the bending the “stronger” the surface The lens power is defined as the reciprocal of the focal length: 1 1 1 D = = (n −1) − f R1 R2 The unit of optical power is the inverse metre or the Diopter symbolised by D How eyes work Accommodation: fine focussing Eye diameter ~25mm How can the lens system be adjusted so that images of objects at different distances are always obtained on the retina? Object far away Object near The answer was first suggested by Hermann von Helmholtz working in Berlin in 1850s. -

Evaluation of Amplitude of Accommodation in a Presbyopic Patient Undergoing Stimulation of Sizhukong Acupuncture Point (Th23)–Case Report
Advances in Ophthalmology & Visual System Case Report Open Access Evaluation of amplitude of accommodation in a presbyopic patient undergoing stimulation of sizhukong acupuncture point (th23)–case report Introduction Volume 8 Issue 1 - 2018 The term “presbyopia” derives from Aristotle’s description about Gilvano Amorim Oliveira observation of persons that could to see well at distance, but poorly at Medicine School PUCCAMP University, Brazil near after 40 years old of age.1 Historically, the term presbyopia was used to describe the condition where the near point has receded too Correspondence: Gilvano Amorim Oliveira, Medicine School far from the eye for normal near-vision tasks. It is the most common PUCCAMP University, Av. Onze de Agosto, no. 736 Sala 22, Vila refractive error in adulthood and is caused by gradual decrease in the Clayton. Valinhos/SP, CEP 13276130, Brazil, amplitude of accommodation,2 observed by about 40 years of age.3 Email Early symptoms are difficulty in focusing on nearby objects under Received: May 12, 2017 | Published: January 19, 2018 inadequate light and at the end of the day and progress to blurred vision at near. Presbyopia can be corrected by wearing multifocal or reading glasses, contact lenses4 or surgery in some cases.1 The physical mechanism of loss of accommodation remains unknown, but Helmholtz’s hypothesis5,6 is one of the most accepted theories that try to explain it. According to the Helmholtz´s model, the contraction of the ciliary muscle would allow relaxation of crystalline capsule, which, and remained orthophoric under cover/uncover test. Biomicroscopy by elastic contraction, would deform the lens, increasing its thickness and fundoscopy were normal.