Assessing the Suitability of the Ujjani Dam Water and Its Surrounding Ground Water for Irrigation Purposes Using GIS Techniques
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Levels Dip in Reservoirs, Alarm Bells Ring
Sep 04 2015 : The Economic Times (Delhi) Water Levels Dip in Reservoirs, Alarm Bells Ring Across States Madhvi Sally New Delhi: Falling water levels in 91 major reservoirs to hit availability for irrigation & drinking The country is heading for a shortage of water for irrigation and drinking as water level in 91 major reservoirs has dipped 16% below normal, and is expected to deplete further with the monsoon on the verge of withdrawal. The Central Water Commission, which monitors reservoirs, plans to issue advisories to states about the situation after the weather office formally announces the date when the monsoon withdraws. Reservoirs usually fill up during the monsoon season, which provides about 75% of the country's rainfall, and supply water for irrigation and drinking for the rest of the year. Officials say the situation is particularly disturbing in western and southern regions although central Indian reservoirs have adequate water. The situation in the north is also better due to heavy downpours in the Himalayan states. AB Pandya, chairman of the Cen tral Water Commission, said the situation is being monitored. “We are waiting for the India Meteorological Department to give the monsoon withdrawal dates. After that we will be in a better situation to come with an advisory,“ he said. The water situation is much worse than last year, when the monsoon began with a severe dry spell of six weeks, but rainfall improved significantly towards the end of the season and filled up reservoirs to optimal levels. This year, the monsoon began strongly but has tapered off in August, leaving reservoirs depleted. -

By Thesis Submitted for the Degree of Vidyavachaspati (Doctor of Philosophy) Faculty for Moral and Social Sciences Department Of
“A STUDY OF AN ECOLOGICAL PATHOLOGICAL AND BIO-CHEMICAL IMPACT OF URBANISATION AND INDUSTRIALISATION ON WATER POLLUTION OF BHIMA RIVER AND ITS TRIBUTARIES PUNE DISTRICTS, MAHARASHTRA, INDIA” BY Dr. PRATAPRAO RAMGHANDRA DIGHAVKAR, I. P. S. THESIS SUBMITTED FOR THE DEGREE OF VIDYAVACHASPATI (DOCTOR OF PHILOSOPHY) FACULTY FOR MORAL AND SOCIAL SCIENCES DEPARTMENT OF SOCIOLOGY TILAK MAHARASHTRA VIDHYAPEETH PUNE JUNE 2016 CERTIFICATE This is to certify that the entire work embodied in this thesis entitled A STUDY OFECOLOGICAL PATHOLOGICAL AND BIOCHEMICAL IMPACT OF URBANISATION AND INDUSTRILISATION ON WATER POLLUTION OF BHIMA RIVER AND Its TRIBUTARIES .PUNE DISTRICT FOR A PERIOD 2013-2015 has been carried out by the candidate DR.PRATAPRAO RAMCHANDRA DIGHAVKAR. I. P. S. under my supervision/guidance in Tilak Maharashtra Vidyapeeth, Pune. Such materials as has been obtained by other sources and has been duly acknowledged in the thesis have not been submitted to any degree or diploma of any University or Institution previously. Date: / / 2016 Place: Pune. Dr.Prataprao Ramchatra Dighavkar, I.P.S. DECLARATION I hereby declare that this dissertation entitled A STUDY OF AN ECOLOGICAL PATHOLOGICAL AND BIO-CHEMICAL IMPACT OF URBANISNTION AND INDUSTRIALISATION ON WATER POLLUTION OF BHIMA RIVER AND Its TRIBUTARIES ,PUNE DISTRICT FOR A PERIOD 2013—2015 is written and submitted by me at the Tilak Maharashtra Vidyapeeth, Pune for the degree of Doctor of Philosophy The present research work is of original nature and the conclusions are base on the data collected by me. To the best of my knowledge this piece of work has not been submitted for the award of any degree or diploma in any University or Institution. -

6. Water Quality ------61 6.1 Surface Water Quality Observations ------61 6.2 Ground Water Quality Observations ------62 7
Version 2.0 Krishna Basin Preface Optimal management of water resources is the necessity of time in the wake of development and growing need of population of India. The National Water Policy of India (2002) recognizes that development and management of water resources need to be governed by national perspectives in order to develop and conserve the scarce water resources in an integrated and environmentally sound basis. The policy emphasizes the need for effective management of water resources by intensifying research efforts in use of remote sensing technology and developing an information system. In this reference a Memorandum of Understanding (MoU) was signed on December 3, 2008 between the Central Water Commission (CWC) and National Remote Sensing Centre (NRSC), Indian Space Research Organisation (ISRO) to execute the project “Generation of Database and Implementation of Web enabled Water resources Information System in the Country” short named as India-WRIS WebGIS. India-WRIS WebGIS has been developed and is in public domain since December 2010 (www.india- wris.nrsc.gov.in). It provides a ‘Single Window solution’ for all water resources data and information in a standardized national GIS framework and allow users to search, access, visualize, understand and analyze comprehensive and contextual water resources data and information for planning, development and Integrated Water Resources Management (IWRM). Basin is recognized as the ideal and practical unit of water resources management because it allows the holistic understanding of upstream-downstream hydrological interactions and solutions for management for all competing sectors of water demand. The practice of basin planning has developed due to the changing demands on river systems and the changing conditions of rivers by human interventions. -

– Kolab River 4)Indravati Dam – Indravati River 5)Podagada Dam – Podagada River 6)Muran Dam – Muran River 7)Kapur Dam – Kapur River
DAMS IN INDIA WEST BENGAL 1)FARRAKA BARRAGE – GANGES RIVER 2)DURGAPUR BARRAGE – DAMODAR RIVER 3)MAITHON DAM –BARAKAR RIVER 4)PANCHET DAM – DAMODAR RIVER 5)KANGSABATI DAM – KANGSABATI RIVER UTTAR PRADESH 1)RIHAND DAM – RIHAND RIVER 2)MATATILA DAM – BETWA RIVER 3)RAJGHAT DAM – BETWA RIVER ODISHA 1)HIRAKUND DAM – MAHANADI 2)RENGALI DAM – BRAHMANI RIVER 3)UPPER KOLAB DAMwww.OnlineStudyPoints.com – KOLAB RIVER 4)INDRAVATI DAM – INDRAVATI RIVER 5)PODAGADA DAM – PODAGADA RIVER 6)MURAN DAM – MURAN RIVER 7)KAPUR DAM – KAPUR RIVER www.OnlineStudyPoints.com DAMS IN INDIA JHARKHAND 1)MAITHON DAM- BARAKAR RIVER 2)PANCHET DAM- DAMODAR RIVER 3)TENUGHAT DAM – DAMODAR RIVER 5)GETALSUD DAM – SWARNAREKHA RIVER MADHYA PRADESH 1)GANDHISAGAR DAM – CHAMBAL RIVER 2)TAWA DAM – TAWA RIVER 3)INDIRA SAGAR DAM – NARMADA RIVER 4)OMKARESHWAR DAM – NARMADA RIVER 5)BARGI DAM – NARMADA RIVER 6)BARNA DAM – BARNA RIVER 7)BANSAGAR DAM – SON RIVER CHHATTISGARH www.OnlineStudyPoints.com 1)MINIMATA BANGO DAM – HASDEO RIVER 2)DUDHWA DAM – MAHANADI 3)GANGREL DAM – MAHANADI 4)SONDUR DAM – SONDUR 5)TANDULA DAM – TANDULA RIVER 6)MONGRA BARRAGE – SHIVNATH www.OnlineStudyPoints.com DAMS IN INDIA MAHARASHTRA 1)KOYNA DAM – KOYNA RIVER 2)JAYAKWADI DAM – GODAVARI RIVER 3)ISAPUR DAM – PENGANA RIVER 4)WARNA DAM – VARNA RIVER 5)TOTLADOH DAM – PENCH RIVER 6)SUKHANA DAM – SUKHANA RIVER 7)UJJANI DAM – BHIMA RIVER JAMMU AND KASHMIR 1)SALAL DAM – CHENAB RIVER 2)BAGLIHAR DAM – CHANAB RIVER 3)PAKUL DUL DAM – CHENAB RIVER 3)URI DAM – JHELUM RIVER 4)NIMBOO BAZGO HYDROELECTRIC PLANT – INDUS RIVER -

Government of Maharashtra Hydrology Project (Sw) Annual Report Water Quality Laboratory Level-Ii Pune Year 2015 Superintending E
FOR OFFICE USE ONLY GOVERNMENT OF MAHARASHTRA WATER RESOURCES DEPARTMENT HYDROLOGY PROJECT (SW) ANNUAL REPORT WATER QUALITY LABORATORY LEVEL-II PUNE YEAR 2015 SUPERINTENDING ENGINEER DATA COLLECTION, PLANNING & HYDROLOGY CIRCLE, NASHIK EXECUTIVE ENGINEER, HYDROLOGY PROJECT DIVISION, PUNE-1 PREFACE Environmental Hydrology in practice primarily restricted to Water Quality Hydrology .A combination of geomorphic ,geologic and soil properties, governs the movement of water from the time it falls on the land surface to till it leaves the surface. With two third of earth surface covered by water and human body consisting 75% of it. It is evidently clear that water is one of the prime elements responsible for life on earth. Water circulates through land just as it does through human body, transporting, dissolving, replenishing, nutrients and organic matter, while carrying away waste material. Human activities in one place are cause of water quality and ecological disturbances elsewhere. Forest degradation is one of the major consequences of long range atmospheric transport and air pollution which also poses an increasing threat to the surface and ground water resources. Pesticides are also becoming widely dispersed within environment and owing to their environmental abilities and persistence over long periods of time are found in eco system either accidently through spray drift agricultural runoff. This report includes water quality data about Rivers Krishna, Bhima and its Tributaries in Maharashtra for the period of June 2014 to May 2015 analyzed by the agency M/s.Papilon Enviro Engineers Pvt. Ltd., Aurangabad and reviewed by Water Resources Department. The contract towards Operation and Maintenance of Water Quality Lab Level-II, Pune for the said period is awarded to above agency. -
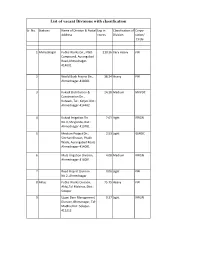
List of Vacant Divisions with Classification
List of vacant Divisions with classification Sr. No. Stations Name of Division & Postal Exp in Classification of Corpo- Address crores Division ration/ Circle 1 Ahmednagar Public Works Dn., PWD 210.36 Very Heavy PW Compound, Aurangabad Road,Ahmednagar- 414001 2 World Bank Project Dn., 38.24 Heavy PW Ahmednagar-414001 3 Kukadi Distribution & 24.38 Medium MKVDC Construction Dn., Kolwadi, Tal : Karjat, Dist : Ahmednagar-414402 4 Kukadi Irrigation Dn. 2.61 Light IRRGN No.II, Shrigonda, Dist : Ahmednagar-413701 5 Medium Project Dn., 2.53 Light GMIDC Sinchan Bhavan, Phakir Wada, Aurangabad Road, Ahmednagar-414001 6 Mula Irrigation Division, 4.08 Medium IRRGN Ahmednagar-414001 7 Road Project Division 0.06 Light PW No.2, Ahmednagar 8 Akluj Public Works Division, 75.75 Heavy PW Akluj,Tal-Malsirus, Dist : Solapur 9 Ujjani Dam Management 0.37 Light IRRGN Division, Bhimanagar, Tal- Madha,Dist : Solapur- 413212 10 Chiplun Irrigation Project 33.25 Medium KIDC Construction Division,Kapsal Vasahat, Chiplun,Dist-Ratnagiri- 415605. 11 Mechanical Dn. for 12.69 Medium IRRGN Konkan Region, Alore, Tal : Chiplun, Dist : Ratnagiri- 415603 12 Construction Dn. (E&M) 6.93 Medium IRRGN No. I, Alore, Tal : Chiplun, Dist : Ratnagiri-415603 13 Quality Control Division, 0.64 Light IRRGN Alore, Chiplun,Dist- Ratnagiri -415603 14 Dhule Sulwade Jamphal Kanoli 136.98 Heavy GMIDC Lift Irrigation Division,Nakane Resorvior Colony,Sakri Road, Dhule- 424002 15 Dhule Medium Project 41.42 Heavy TIDC Dn. ,Sinchan Bhavan, Sakri Road, Dhule-424001 16 Dhule Irrigation Dn., 1.71 Light IRRGN Sinchan Bhavan,Sakri Road, Dhule-424001 17 Quality Control Dn., 0.12 Light IRRGN Sinchan Bhavan, Sakri Road, Dhule-424002 18 Dhule Electrical 0 Light PW Division,Dhule. -

Government of Maharashtra Hydrology Project (Sw) Annual Report Water Quality Laboratory Level-Ii Pune Year 2012 Superintending E
FOR OFFICE USE ONLY GOVERNMENT OF MAHARASHTRA WATER RESOURCES DEPARTMENT HYDROLOGY PROJECT (SW) ANNUAL REPORT WATER QUALITY LABORATORY LEVEL-II PUNE YEAR 2012 SUPERINTENDING ENGINEER DATA COLLECTION, PLANNING & HYDROLOGY CIRCLE, NASHIK EXECUTIVE ENGINEER, HYDROLOGY PROJECT DIVISION, PUNE-1 PREFACE Water quality has become an important issue for policy makers in the Government both at the Central and the State level. Water is for sure the only common and global issue that interests all the living bodies of the world including humans, flora and fauna. Without water, survival is not possible. The two components of water are namely quality and quantity. As the amount of usable water differs from country to country, place to place, season to season, water shortage is experienced in some countries. It is well known that water resources are not equally distributed according to population densities among the countries. Water resources are polluted especially through human induced activities. Another portion of water is lost during transmission due to lack of efficient and proper infrastructure. Thus, pollution of water resources leads to serious sanitary problems. 1/5th of the world’s population that accounts to approximately 1.1 billion people is under the risk of water related diseases, and moreover each year many people die as they do not have safe drinking water. The main reason of this fact is lack of adequate infrastructure rather than water scarcity. This report includes water quality data about Rivers Krishna, Bhima and its Tributaries in Maharashtra for the period of June 2011 to May 2012 analyzed by the agency M/s. -

Distribution of Sediments in the Reservoir by Area Reduction Method
International Journal of Engineering and Advanced Technology (IJEAT) ISSN: 2249-8958, Volume-8 Issue-5, June 2019 Distribution of Sediments in the Reservoir by Area Reduction Method P. P. Mahadik, P. T. Nimbalkar, R. H. Jadhav in, or deposited by water or air, or accumulated in the beds by Abstract: The repository of water for irrigation, water supply other natural agents; any detritus accumulation, such as and power generation or for moderation of floods necessitates the loess. Sediment is also sometimes known as alluvium. The construction of dams across rivers. As a consequence of the branch of engineering which deals with rivers and canals reduced velocity upstream of the dam all the bed load and a part of the suspended load is deposited in the quiet pool of water in the flowing through loose transportable material (sediment or reservoir. This reduces the capacity of the reservoir for storing alluvium) and transporting some of it along with water is water by depositing the sediment in therein. There are two main called fluvial hydraulics, sediment engineering or alluvial methods of forecast of sediment distribution are area reduction river dynamics. method and area increment respectively. For the prediction of Sediment engineering deals with nature of sediment Sediment distribution in dam’s reservoir we can use the area problems, detailed study of sediment deposition in reservoir, reduction method & by using the Borland and Miller method distribution of sediments. For sediment deposition and finding out its parameters (C,m,n).the main objective of Borland and Miller technique is to determine depth/capacity /surface area distribution area reduction method is used. -

The Status Report
WATER RESOURCES DEPARTMENT GOVERNMENT OF MAHARASHTRA THE STATUS REPORT CAPACITY ASSESSMENT OF RESERVOIRS IN MAHARASHTRA [ 1974 - 2020 ] JAYAKWADI DAM MAHARASHTRA ENGINEERING RESEARCH INSTITUTE ( M. E. R. I. ) RESOURCES ENGINEERING CENTER NASHIK - 04 UJJANI DAM YEAR 2020 RESOURCES ENGINEERING CENTER, MERI, NASHIK - 04 A Publication of MERI, Nashik (MS) A Publication of MERI, Nashik (MS) THE STATUS REPORT CAPACITY ASSESSMENT OF RESERVOIRS IN MAHARASHTRA SURVEY FROM 1974 TO 2020 MAHARASHTRA ENGINEERING RESEARCH INSTITUTE ( M. E. R. I. ) , NASHIK – 04 RESOURCES ENGINEERING CENTER NASHIK - 04 Resources Engineering Center, MERI , Nashik I FOREWARD The sedimentation is an effect of erosion in the catchment area of the reservoir and hence, lesser the rate of erosion, smaller is the sediment load entering into the reservoir. Deposition of sedimentation in reservoir gradually reduces the capacity of reservoir. Dead storage capacity as well as live storage capacity gets affected due to sedimentation. Information about reduction in the storage capacity is necessary for the planning and operational purposes and same can be obtained through sediment assessment surveys done at regular interval. Various factors govern the process of erosion, transport and deposition of sediment in the reservoir. Type of soil, drainage density, vegetation, rainfall intensity and duration, shape of catchment and land use / land cover affect erosion. Sediment transportation depends upon slope of the catchment, channel geometry and nature of river bank and bed. Deposition is a function of bed slope of the reservoir, length of reservoir, flow patterns, inflow-outflow rates, grain size distribution, mode of reservoir operation etc. Established in 1959, The Maharashtra Engineering Research Institute (MERI), Nashik, is a premier engineering research institute working in the field of applied research in civil engineering. -

PI.ANNING DEPARTMENT =}= 0 RA R T . ANNUALPLAN
h i t l%t% If * * * I CQUERNMENT Of MAHARASHTRA ^ |. * ?. ?. * ^ * PI.ANNING DEPARTMENT =}= t % ^ * * :>;< '> ?,i ^ ?jc ^ 0 R A r T . ANNUAL PLAN % | I J j - ---------1980-81 Ii ♦* r$ $ . *■* .;; * % DECEMBER 1979 >1: * .'}= ;«I* I * J 1: S '£ J * l l H it T ^ ^ * J * * « i« r / . ^ J NIEPA DC D00051 Draft Annual Plan 1980-81- Maharashtra State. Contents, Statements Pages li Abstract statement- State plan GNl 1-9 2d Abstract Sta tement- K'4NP GN4 1 3d Abstract Statement-Centrally GN6 1 Programme,, Agril.Resea rch and Education Agril. 1 18 5^ C^op. Husbandry Agri. 19-41 Land Reforms l r 1 . 7} Drought Prone Areas Programme DPAP 1-2 8) Warehousing and Marketing WM 1 9) Minor Irrigation MI- 1-3 10)Soil Conservation SC 1-4 11) Ayacut Development AD-1-2 12) Command Area Development CAD 1'-^^, 13) An.inal Hus'oandry A.H.1-10 14) Dairy Development DD 1-11 15) Fisheries PISH 1-6 16) Forests FOR 1-5 17)Investment in A^ril.financial Ip^t, . ACl 18)Community Development CDl 19)Integrated Rural Development IRD 1-3 20) Cooperation Coop.1-11 21) larrigation .iiiR 1-92 S 2) Power POW 1-33 23) Industry and Mining IM-1-9' 24) Transport and Communications TC 1-8 25) General Education I ED 1-39 26) Art and Culture X , 27) Technical EducatiorjC 2S) Health HEL l-2f 25) Nutrition ‘ NUT lr-3 3D) Sewera ge and Water Supply WS 1 - 7 31) Housing HS 1-5 32) Urban Development UD 1-3 33) Information and P\iblicity INP 1-2 34) Labour and Labour VJelfa re L & LW 1-6 35) Welfare of Backward Classes BCW 1-14 36) Social Welfare SW 1-9 3") Emplo^TTient Guarantee Scheme BGS 38) Other Programmes STS 1-4 G l\l-l ( Con td .) (Rs. -

A Case Study of Ujjani Reservoir in Maharashtra, India
International Journal of Civil Engineering and Technology (IJCIET) Volume 9, Issue 1, January 2018, pp. 245–252, Article ID: IJCIET_09_01_025 Available online at http://http://iaeme.com/Home/issue/IJCIET?Volume=9&Issue=1 ISSN Print: 0976-6308 and ISSN Online: 0976-6316 © IAEME Publication Scopus Indexed SWAT MODELLING FOR SEDIMENT YIELD: A CASE STUDY OF UJJANI RESERVOIR IN MAHARASHTRA, INDIA R. B. Shendge, Dr. M. P. Chockalingam, B. Saritha and A. Ambica Department of Civil Engineering, Bharath University, Chennai, India ABSTRACT This study is related to the use of SWAT model for the prediction of sediment yield from the rainwater runoff in the Upper Bhima Basin (UBB) which is the source of water for the terminal reservoir called Ujjani Reservoir, in Solapur district, Maharashtra State, in India. SWAT-model configuration was developed, using data pertaining to 27 sub-basins in the catchment area of the UBB. Extensive use of satellite images has been done, for a period of 2 years and 7 months (January 2012 to July 2014). Digital Elevation Model (DEM), Land Use and Soil data were included, in addition to climatic data applicable to the area. The annual average sediment yield in the various reaches of sub-basins was estimated to vary from 1342.0 tons/year to 888010.0 tons/year, during the study period. The trend of results the SWAT model applied to Ujjani Reservoir will serve as a clue to similar studies in major reservoirs in India, in estimating the sediment yield from the rainwater surface runoff. These estimates have relevance to soil conservation schemes envisioned in the catchment areas, and for ensuring adequate water storage needed for meeting the requirements of the various human uses. -

Crop Diversification in Pune District: a Geographical Analysis
Scholarly Research Journal for Humanity Science & English Language, Online ISSN 2348-3083, SJIF 2016 = 4.44, www.srjis.com UGC Approved Sr. No.48612, AUG-SEPT 2017, VOL- 4/23 CROP DIVERSIFICATION IN PUNE DISTRICT: A GEOGRAPHICAL ANALYSIS Wayal Navnath Laxman Assistant Professor, Department of Geography, Modern College of Arts, Science and Commerce, Shivajinagar, Pune (Maharashtra). Agriculture is one of the ancient techniques of growing crops in the world. Crop diversification means to raise a variety of crops in particular agriculture region. Crop diversification usually leads to changes in the traditional cropping pattern of the agricultural region. Crop diversification is exact opposite of crop specialization, which gives a wider choice for production of variety of crops in any region and increases production related activities. Crop diversification is collectively reflection of physio-climatic, socio-economic and techno-organization inputs. Regional development can be achieved through agricultural development. Agricultural development can achieved through proper agricultural planning and well-designed policies and their implementation. To determine planning strategy for regional agricultural development, study of various aspects of agricultural regionalization is essential. Crop diversification is one of the laying path for the study of agricultural regionalization. Therefore, present study is focused analytical study of crop diversification in Pune district. This study is to examine the tahsil-wise spatio-temporal changes in crop diversification of Pune district during 2001 to 2016. Pune district is an agriculturally pre-dominant district in Maharashtra. It has attained spatio-temporal changes in cropping pattern accordingly development in irrigation facilities, market, transportation, infrastructure and technology. Crop diversification have been computed on the basis of Crop Diversification Index method initiated by Bhatia.