Cascades Fisher Reintroduction Project: Progress Report for April 2019 to June 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1922 Elizabeth T
co.rYRIG HT, 192' The Moootainetro !scot1oror,d The MOUNTAINEER VOLUME FIFTEEN Number One D EC E M BER 15, 1 9 2 2 ffiount Adams, ffiount St. Helens and the (!oat Rocks I ncoq)Ora,tecl 1913 Organized 190!i EDITORlAL ST AitF 1922 Elizabeth T. Kirk,vood, Eclttor Margaret W. Hazard, Associate Editor· Fairman B. L�e, Publication Manager Arthur L. Loveless Effie L. Chapman Subsc1·iption Price. $2.00 per year. Annual ·(onl�') Se,·ent�·-Five Cents. Published by The Mountaineers lncorJ,orated Seattle, Washington Enlerecl as second-class matter December 15, 19t0. at the Post Office . at . eattle, "\Yash., under the .-\0t of March 3. 1879. .... I MOUNT ADAMS lllobcl Furrs AND REFLEC'rION POOL .. <§rtttings from Aristibes (. Jhoutribes Author of "ll3ith the <6obs on lltount ®l!!mµus" �. • � J� �·,,. ., .. e,..:,L....._d.L.. F_,,,.... cL.. ��-_, _..__ f.. pt",- 1-� r�._ '-';a_ ..ll.-�· t'� 1- tt.. �ti.. ..._.._....L- -.L.--e-- a';. ��c..L. 41- �. C4v(, � � �·,,-- �JL.,�f w/U. J/,--«---fi:( -A- -tr·�� �, : 'JJ! -, Y .,..._, e� .,...,____,� � � t-..__., ,..._ -u..,·,- .,..,_, ;-:.. � --r J /-e,-i L,J i-.,( '"'; 1..........,.- e..r- ,';z__ /-t.-.--,r� ;.,-.,.....__ � � ..-...,.,-<. ,.,.f--· :tL. ��- ''F.....- ,',L � .,.__ � 'f- f-� --"- ��7 � �. � �;')'... f ><- -a.c__ c/ � r v-f'.fl,'7'71.. I /!,,-e..-,K-// ,l...,"4/YL... t:l,._ c.J.� J..,_-...A 'f ',y-r/� �- lL.. ��•-/IC,/ ,V l j I '/ ;· , CONTENTS i Page Greetings .......................................................................tlristicles }!}, Phoiitricles ........ r The Mount Adams, Mount St. Helens, and the Goat Rocks Outing .......................................... B1/.ith Page Bennett 9 1 Selected References from Preceding Mount Adams and Mount St. -

Kaiser Permanente CORE Provider List
Core Plans Provider Directory Table of Contents Personal Physicians 1 (1926 Total) Specialty Care 27 (7979 Total) Behavioral Health Services 170 (2922 Total) Urgent Care 225 (85 Total) Hospitals 228 (69 Total) Pharmacies 231 (283 Total) Other Facilities 239 (848 Total) Kaiser Permanente Washington Medical Centers 261 (25 Total) Index 262 Contact Information back cover kp.org/wa | 1-888-901-4636 | All plans offered and underwritten by Kaiser Foundation Health Plan of Washington i Personal Physicians ADOLESCENT MEDICINE Skagit Regional Health - Arlington Family Bellingham Bay Family Medicine - cont. Medicine 722 N State St 7530 204th St NE (360) 752-2865 Olympia (360) 435-8810 Bowling, Sara Ashley, MD Chaffee, Charles T, MD Fox, Laura Vh, DO Kaiser Permanente Olympia Medical Center Evans, Sarah M, ARNP Hopper, James G, MD 700 Lilly Rd NE Lucianna, Mark A, MD O'Keefe, Karen Davis, MD (360) 923-7000 Schimke, Melana K, MD Skagit Regional Health - Arlington Pediatrics Van Hofwegen, Lisa Marie, MD 875 Wesley St Ste 130 Bellingham Family and Women's Health (360) 435-6525 1116 Key St Ste 106 Kraft, Kelli Malia, ARNP (360) 756-9793 Wood, Franklin Hoover, MD Whitehorse Family Medicine Kopanos, Taynin Kay, ARNP Sprague, Bonnie L, ARNP 875 Wesley St Ste 250 Spokane (360) 435-2233 Bellingham Family Medicine Fletcher, James Rodgers, MD MultiCare Rockwood Main 12 Bellwether Way Ste 230 Janeway, David W, MD (360) 738-7988 400 E 5th Ave Myren, Karen Sue, MD Nuetzmann, John S, DO (509) 838-2531 Carey, Alexandra S, MD Bellevue Fairhaven Family & Sports Medicine -

Cascades Inc
PRESS RELEASE Cascades Inc. Telephone: 819-363-5100 404 Marie-Victorin Blvd., P.O. Box 30 Fax: 819-363-5155 Kingsey Falls, Quebec J0A 1B0 Canada www.cascades.com CASCADES ACQUIRES CONTAINERBOARD PACKAGING PLANTS IN ONTARIO AND INCREASES ITS OWNERSHIP IN GREENPAC Kinsey Falls, QC, December 4, 2017 – Cascades Inc. (TSX: CAS) announces the acquisition of four plants in Ontario to strengthen its position in the containerboard packaging sector, and the purchase of an ownership position in Tencorr Holdings Corporation (“Tencorr”). The company also announces an increase in its equity holding of the Greenpac Mill LLC (“Greenpac”). “We are very pleased to expand our presence in Ontario and increase our stake in Greenpac for the second time this year. These transactions align perfectly with our vision and strategy for our containerboard activities,” stated Cascades President and CEO Mario Plourde. The four following plants were acquired from the Coyle family, and specialize in the manufacturing of boxes and speciality products offering strong growth potential: - McLeish Corr-a-Box Packaging & Design – Etobicoke, Ontario - Brown Packaging – Burlington, Ontario - Coyle Corrugated Containers Inc. – Scarborough, Ontario - Coyle Packaging (Peterborough) Ltd. – Peterborough, Ontario This transaction will allow Cascades to expand its presence in Ontario, to increase its production capacity by 500 million square feet per year, and to strengthen its ability to serve customers in this region. These plants already have procurement agreements with Greenpac, and as such the transaction will have little impact on Cascades’ integration rate. Cascades has also acquired the Coyle family’s 33% stake in Tencorr, a company specialized in manufacturing sheet stock for box producers. -

New Titles for Spring 2021 Green Trails Maps Spring
GREEN TRAILS MAPS SPRING 2021 ORDER FORM recreation • lifestyle • conservation MOUNTAINEERS BOOKS [email protected] 800.553.4453 ext. 2 or fax 800.568.7604 Outside U.S. call 206.223.6303 ext. 2 or fax 206.223.6306 Date: Representative: BILL TO: SHIP TO: Name Name Address Address City State Zip City State Zip Phone Email Ship Via Account # Special Instructions Order # U.S. DISCOUNT SCHEDULE (TRADE ONLY) ■ Terms: Net 30 days. 1 - 4 copies ........................................................................................ 20% ■ Shipping: All others FOB Seattle, except for orders of 25 books or more. FREE 5 - 9 copies ........................................................................................ 40% SHIPPING ON BACKORDERS. ■ Prices subject to change without notice. 10 - 24 copies .................................................................................... 45% ■ New Customers: Credit applications are available for download online at 25 + copies ................................................................45% + Free Freight mountaineersbooks.org/mtn_newstore.cfm. New customers are encouraged to This schedule also applies to single or assorted titles and library orders. prepay initial orders to speed delivery while their account is being set up. NEW TITLES FOR SPRING 2021 Pub Month Title ISBN Price Order February Green Trails Mt. Jefferson, OR No. 557SX 9781680515190 18.00 _____ February Green Trails Snoqualmie Pass, WA No. 207SX 9781680515343 18.00 _____ February Green Trails Wasatch Front Range, UT No. 4091SX 9781680515152 18.00 _____ TOTAL UNITS ORDERED TOTAL RETAIL VALUE OF ORDERED An asterisk (*) signifies limited sales rights outside North America. QTY. CODE TITLE PRICE CASE QTY. CODE TITLE PRICE CASE WASHINGTON ____ 9781680513448 Alpine Lakes East Stuart Range, WA No. 208SX $18.00 ____ 9781680514537 Old Scab Mountain, WA No. 272 $8.00 ____ ____ 9781680513455 Alpine Lakes West Stevens Pass, WA No. -

Mt. Baker Ski Area
Winter Activity Guide Mount Baker Ranger District North Cascades National Park Contacts Get ready for winter adventure! Head east along the Mt. Baker Mt. Baker-Snoqualmie National Forest State Road Conditions: /Mt. Baker Ranger District Washington State Dept. of Transportation Highway to access National Forest 810 State Route 20 Dial 511 from within Washington State lands and the popular Mt. Baker Ski Sedro-Woolley, WA 98284 www.wsdot.wa.gov Area. Travel the picturesque North (360) 856-5700 ext. 515 Glacier Public Service Center Washington State Winter Recreation and Cascades Highway along the Skagit 10091 Mt. Baker Highway State Sno-Park Information: Wild & Scenic River System into the Glacier, WA 98244 www.parks.wa.gov/winter heart of the North Cascades. (360) 599-2714 http://www.fs.usda.gov/mbs Mt. Baker Ski Area Take some time for winter discovery but North Cascades National Park Service Ski Area Snow Report: be aware that terrain may be challenging Complex (360) 671-0211 to navigate at times. Mountain weather (360) 854-7200 www.mtbaker.us conditions can change dramatically and www.nps.gov/noca with little warning. Be prepared and check Cross-country ski & snowshoe trails along the Mt. Baker Highway: forecasts before heading out. National Weather Service www.weather.gov www.nooksacknordicskiclub.org Northwest Weather & Avalanche For eagle watching information visit: Travel Tips Center: Skagit River Bald Eagle Interpretive Center Mountain Weather Conditions www.skagiteagle.org • Prepare your vehicle for winter travel. www.nwac.us • Always carry tire chains and a shovel - practice putting tire chains on before you head out. -

Source of Possibilities 3 Alain Lemaire Executive Chairman of the Board of Directors
2019 ANNUAL REPORT 2019 CASCADES Source of Possibilities 2019 Annual Report 2019 at a Glance Containerboard Packaging 49th A Canadian leader Global 100 Most Sustainable 6th largest producer in North America Corporations in the World (Corporate Knights) Specialty Products A North American leader in industrial and food packaging 6th A leading North American producer of honeycomb paperboard Canada’s top 50 corporate citizens (Corporate Knights) Tissue Papers A leader in tissue papers production in Canada 4th largest producer in North America 9th consecutive year Recovery most responsible company A Canadian leader in the recovery of recycled fibres and brand according to Quebecers, as measured by the Barometer of Responsible Consumption Boxboard Europe1 2nd largest producer of coated recycled boxboard in Europe $578 M $4,996 M $547 M $604 M invested in property, plant & 2 2 equipment, business acquisitions Sales OIBD Adjusted OIBD and in our management systems, excluding right-of-use assets 3.4 M 84% 77% short tons of recycled fibre saved of the fibre used to make of our manufacturing from landfills in North America and our products is recycled waste is reused Europe, for all the Corporation 1.7 3 14% 50% 26% OSHA rate reduction in our energy reduction in our greenhouse reduction in our consumption since 2010 gas emissions intensity water consumption since 1990* since 2010 1 Via our 57.95% equity ownership in Reno de Medici S.p.A. (at Dec. 31, 2019), a public Italian company. 2 Please refer to the “Forward-looking Statements” and “Supplemental Information on Non-IFRS Measures’’ sections for more details. -

Expedition Descriptions
BOLD & GOLD EXPEDITION DESCRIPTIONS BOYS AND GIRLS OUTDOOR LEADERSHIP DEVELOPMENT AT THE YMCA OF GREATER SEATTLE BOLD & GOLD EXPEDITION DESCRIPTIONS 1 WELCOME FROM THE BOLD & GOLD TEAM! To Our Old and New Friends, Welcome to our community! You have taken the first step to discovering what you are truly capable of. BOLD & GOLD is a program that will guide you to find the strength in yourself, in the community around you and in the outdoors. Whether it is exploring the old growth forest of North Cascades National Park, backpacking along the wild coastline of Olympic National Park, or summiting Mount Baker, you will have the opportunity to explore the beauty of nature, overcome challenges, try new things, and create lifelong friendships. We applaud you for taking the first step. While navigating the challenges of travel in the wilderness, we will help you embrace multicultural leadership by combining your unique self and our program’s values. You now have the chance to live beyond your wildest dreams! Thank you for seizing this opportunity and we look forward to hearing your stories when you return. In the words of Dr. Seuss: “You’re off to Great Places! Today is your day! Your mountain is waiting, So... get on your way!” - See you soon! The BOLD & GOLD Team TABLE OF CONTENTS BOLD | 1-WEEK EXPEDITIONS 3 MAKE A SCENE: ART AND BACKPACKING IN THE NORTH CASCADES . 13 BACKPACKING AND FISHING IN THE NORTH CASCADES . 3 POETS AND PEAKS: EXPLORING WILD PLACES THROUGH BEYOND CITY LIMITS: CAMPING AND HIKING AT MT. RAINIER CREATIVE WRITING . -

Evaluation of a National Seasonal Snowfall Record at the Mount Baker, Washington, Ski Area
EVALUATION OF A NATIONAL SEASONAL SNOWFALL RECORD AT THE MOUNT BAKER, WASHINGTON, SKI AREA Robert J. Leffler, Andrew Horvitz and Raymond Downs National Weather Service Office of Climate, Water, and Weather Services Silver Spring, Maryland Michael Changery National Climatic Data Center (NCDC) [retired] Asheville, North Carolina Kelly T. Redmond Desert Research Institute Western Regional Climate Center (WRCC) Reno, Nevada George Taylor Oregon Climate Service Oregon State University Corvallis, Oregon Abstract tion. For this event, the chairman was Robert J. Leffler. The Committee also included Kelly Redmond (Western During the 1998-99 July-June snowfall season, the Regional Climate Center) and Raymond Downs (NWS Mount Baker Ski Area in northwest Washington state Office of Climate, Water, and Weather Services). The com reported 1,140 inches of snowfall. As the season progressed prehensive evaluation leading to the recommendation that and heavy snow continued to fall, the ski area became a new record was set is discussed herein. The problems in increasingly aware of the potential to exceed the existing measuring snow depth are also reiterated. and accepted record of 1,122 inches, set in 1971-72 at the National Weather Service (NWS) cooperative observing sta tion at Paradise Ranger Station on the southern slopes of 1. Introduction Mount Rainier 150 miles to the south. The 1971-72 snow fall was first published as a new US. record by the editor of Some of the heaviest seasonal snowfall totals in the Weatherwise Magazine (Ludlum 1972). Later that year, the United States and the world have been recorded in the value was also recognized by the NWS (Ruscha 1972). -
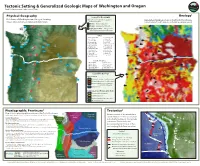
Tectonic Setting & Generalized Geologic Maps of Washington And
on the Lea rs din e g ch E a . d e . g T e Tectonic Setting & Generalized Geologic Maps of Washington and Oregon The TO TLE Pict Earthquakes ure Frank D. Granshaw and Jenda Johnson, 2009 Volcanoes Tsunamis Geology1 Physical Geography Legend for Geography Relief map of Washington and Oregon showing Map colors correspond to vegetation Generalized geologic map of the Pacific Northwest. major cities, volcanoes, lakes and waterways. and surface types: (Circled numbers are the same as on Physical Geography legend) Greens—dense vegetation 1 Browns—grasslands and desert 1 A A Light gray to white—unvegetated alpine regions in the Cascade Range 2 2 Cities A Bellingham, WA N La Grande, OR B Seattle, WA O Baker, OR B I C Tacoma, WA P Ontario, OR B I D Olympia, WA B Bend, OR E Aberdeen, WA R Klamath Falls, OR C H C F Ellensberg, WA S Medford, OR E D 3 F G Yakima, WA T Coos Bay, OR D 3 H Wenatchee, WA U Newport, OR I Spokane, WA V Astoria ,OR G J Walla Walla, WA W Portland, OR G K Pasco, WA X Salem, OR K 4 4 5 L The Dalles, OR Y Eugene, OR 5 V V M Pendleton, OR J J Cascade Volcanoes M M L L W W 1 Mount Baker 6 Mount Hood 6 6 2 Glacier Peak 7 Mount Jeerson N N 3 Mount Rainier 8 Three Sisters 4 Mount St. Helens 9 Newberry Volcano X X 5 Mount Adams 10 Crater Lake O 7 7 Legend for Geology U U Igneous Rock 8 8 Q Q Y Y P Volcanic (Less than 1.6 million years) Volcanic (More than 1.6 million years) 9 9 Volcanic (Mostly 16.5- to 6-million- year-old flood basalts) T T Intrusive rock 10 Sedimentary and Metamorphic Rock 10 Unconsolidated sediment Sedimentary rock S Sedimentary & metamorphic rock R S R Metamorphic rock Physiographic Provinces2 Tectonics3 Map of major physiographic provinces in the Pacic Northwest. -

Washington State's Scenic Byways & Road Trips
waShington State’S Scenic BywayS & Road tRipS inSide: Road Maps & Scenic drives planning tips points of interest 2 taBLe of contentS waShington State’S Scenic BywayS & Road tRipS introduction 3 Washington State’s Scenic Byways & Road Trips guide has been made possible State Map overview of Scenic Byways 4 through funding from the Federal Highway Administration’s National Scenic Byways Program, Washington State Department of Transportation and aLL aMeRican RoadS Washington State Tourism. waShington State depaRtMent of coMMeRce Chinook Pass Scenic Byway 9 director, Rogers Weed International Selkirk Loop 15 waShington State touRiSM executive director, Marsha Massey nationaL Scenic BywayS Marketing Manager, Betsy Gabel product development Manager, Michelle Campbell Coulee Corridor 21 waShington State depaRtMent of tRanSpoRtation Mountains to Sound Greenway 25 Secretary of transportation, Paula Hammond director, highways and Local programs, Kathleen Davis Stevens Pass Greenway 29 Scenic Byways coordinator, Ed Spilker Strait of Juan de Fuca - Highway 112 33 Byway leaders and an interagency advisory group with representatives from the White Pass Scenic Byway 37 Washington State Department of Transportation, Washington State Department of Agriculture, Washington State Department of Fish & Wildlife, Washington State Tourism, Washington State Parks and Recreation Commission and State Scenic BywayS Audubon Washington were also instrumental in the creation of this guide. Cape Flattery Tribal Scenic Byway 40 puBLiShing SeRviceS pRovided By deStination -

Women in Leadership at S&P/Tsx Companies
WOMEN IN LEADERSHIP AT S&P/TSX COMPANIES Women in Leadership at WOMEN’S S&P/TSX Companies ECONOMIC Welcome to the first Progress Report of Women on Boards and Executive PARTICIPATION Teams for the companies in the S&P/TSX Composite Index, the headline AND LEADERSHIP index for the Canadian equity market. This report is a collaboration between Catalyst, a global nonprofit working with many of the world’s leading ARE ESSENTIAL TO companies to help build workplaces that work for women, and the 30% Club DRIVING BUSINESS Canada, the global campaign that encourages greater representation of PERFORMANCE women on boards and executive teams. AND ACHIEVING Women’s economic participation and leadership are essential to driving GENDER BALANCE business performance, and achieving gender balance on corporate boards ON CORPORATE and among executive ranks has become an economic imperative. As in all business ventures, a numeric goal provides real impetus for change, and our BOARDS collective goal is for 30% of board seats and C-Suites to be held by women by 2022. This report offers a snapshot of progress for Canada’s largest public companies from 2015 to 2019, using the S&P/TSX Composite Index, widely viewed as a barometer of the Canadian economy. All data was supplied by MarketIntelWorks, a data research and analytics firm with a focus on gender diversity, and is based on a review of 234 S&P/TSX Composite Index companies as of December 31, 2019. The report also provides a comparative perspective on progress for companies listed on the S&P/TSX Composite Index versus all disclosing companies on the TSX itself, signalling the amount of work that still needs to be done. -

Mount Baker View NEWS of the MOUNT BAKER COMMUNITY CLUB Issue 236, August 2013 2811 Mt
The Mount Baker View NEWS OF THE MOUNT BAKER COMMUNITY CLUB ISSUE 236, AUGUST 2013 2811 Mt. Rainier Drive S. | www.mountbaker.org | 206.722.7209 TRANSIT FORUM PUTS NEIGHBORS AT THE CENTER OF MOUNT BAKER STATION DEVELOPMENT By Sue Cary, outgoing MBCC Zoning, Land Use, Planning & Transportation (ZLUPT) Committee Chair On April 8, over 100 people gathered at the Clubhouse for a com- City Council members Richard Conlin, Tom Rasmussen and Sally munity forum on the issues and opportunities surrounding new Clark were present, along with representatives from Sound Transit, development in the vicinity of the Mount Baker light rail station. King County Metro, University of Washington, South East Effective The purpose of the forum, in keeping with the mission of the Com- Development, Seattle Department of Transportation, Seattle Office munity Club, was to inform the community regarding related pub- of Housing, Rainier Valley Community Loan Fund, Artspace and lic policy issues and provide a setting for civic discussion of the other developers and owners of nearby commercial properties. issues. This forum was the culmination of almost three years of community participation in the City’s planning process for transit- Before opening the discussion to general questions from the audi- oriented development and proposed zoning changes. ence, Andy directed a number of questions to our panelists deal- ing with development opportunities, impediments to quality new Incoming Board President Andy Reynolds moderated a panel development, traffic and access issues.