09 Chapter 04.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Most Lasting Impact of the Imperial Rule in the Jalpaiguri District
164 CHAPTER 111 THE BRITISH COLONIAL AUTHORITY AND ITS PENETRATION IN THE CAPITAL MARKET IN THE NORTHERN PART OF BENGAL The most lasting impact of the imperial rule in the Jalpaiguri District especially in the Western Dooars was the commercialisation of agriculture, and this process of commercialisation made an impact not only on the economy of West Bengal but also on society as well. J.A. Milligan during his settlement operations in the Jalpaiguri District in 1906-1916 was not im.pressed about the state of agriculture in the Jalpaiguri region. He ascribed the backward state of agriculture to the primitive mentality of the cultivators and the use of backdated agricultural implements by the cultivators. Despite this allegation he gave a list of cash crops which were grown in the Western Duars. He stated, "In places excellent tobacco is grown, notably in Falakata tehsil and in Patgram; mustard grown a good deal in the Duars; sugarcane in Baikunthapur and Boda to a small extent very little in the Duars". J.F. Grunning explained the reason behind the cultivation of varieties of crops in the region due to variation in rainfall in the Jalpaiguri district. He said "The annual rainfall varies greatly in different parts of the district ranging from 70 inches in Debiganj in the Boda Pargana to 130 inches at Jalpaiguri in the regulation part of the district, while in the Western Duars, close to the hills, it exceeds 200 inches per annum. In these circumstances it is not possible to treat the district as a whole and give one account of agriculture which will apply to all parts of it".^ Due to changes in the global market regarding consumer commodity structure suitable commercialisation at crops appeared to be profitable to colonial economy than continuation of traditional agricultural activities. -

3 an EMPIRICAL STUDY ON.Cdr
IJRTBT AN EMPIRICAL STUDY ON ECONOMIC DEVELOPMENT OF TEA INDUSTRY IN WEST BENGAL Indranil Chatterjee1*, Amit Majumdar2 1Mewar University, Rajasthan, India 2Department of Commerce, Bijoy Krishna Girls College, Howrah, India *Corresponding Author's Email: [email protected] ABSTRACT Introduction: Economic Development is the primary issue of any developed/developing countries. Economic growth of any country depends on the slandered of living for its countrymen. It could be measured which may be taken under a framed structure through adoption of updating technologies from agricultural field to industry field. Literature Review: Tea Industry is a second foreign exchange earner in India. More than 50% women workers are employed in tea estates. Being most of the tea gardens are situated in the remote corner of the various States in India, tea industry is always trying to holistic development of their workers as well as local people. Tea industry is an agricultural-based industry which had already recognised by RBI (Reserve Bank of India). Research Methodology: The study has covered two tea estates in West Bengal. Primary Information is collected through interviews by preparing questionnaire. Secondary Information is collected from newspapers, Journals and public domain. Results & Discussion: (i) In Tea industry more than 50% workers are women. (ii) Tea Industry is a second foreign exchange earner in India. (iii) After Railway & Armed Force, tea Industry is the largest employer in India, and (iv) Being tea gardens are situated in the remote corners of the country, tea industry has already taken various steps to up- lift their workers as well as local people. Conclusion: In this paper has been structured to analyse how the tea industry fulfil their task towards their economic development for their workers and looked out to analyse their performance towards their contribution in the society. -
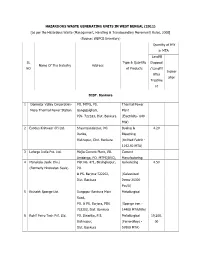
Hazardous Waste Generating Units in West Bengal (2011
HAZARDOUS WASTE GENERATING UNITS IN WEST BENGAL (2011) [as per the Hazardous Waste (Management, Handling & Transboundary Movement) Rules, 2008] (Source: WBPCB Inventory) Quantity of HW in MTA Landfill SL Type & Quantity Disposal Name Of The Industry Address NO of Products / Landfill Inciner After ation Treatme nt DIST. Bankura 1 Damodar Valley Corporation- PO. MTPS, PS. Thermal Power Mejia Thermal Power Station. Gangajalghati, Plant PIN- 722183, Dist. Bankura (Electricity- 840 MW) 2 Exodus Knitwear (P) Ltd. Shyamsundarpur, PO. Dyeing & 4.20 Darika, Bleaching Bishnupur, Dist. Bankura (Knitted Fabric - 1192.92 MTA) 3 Lafarge India Pvt. Ltd. Mejia Cement Plant, Vill. Cement Amdanga, PO. MTPS(DVC), Manufacturing 4 Manaksia (Galv. Div.) PIN.Plot No.722183 471, Birsinghapur, PortlandGalvanizing Cement 4.50 (Formerly Hindustan Seals) PO. - 83330 MTA) & PS. Barjora-722202, (Galvanized Dist. Bankura Items-36000 Pcs/A) 5 Rishabh Sponge Ltd. Durgapur Bankura Main Metallurgical Road, PO. & PS. Barjora, PIN. (Sponge iron - 722202, Dist. Bankura 14400 MTA/Kiln) 6 Rohit Ferro Tech Pvt. Ltd. PO. Dwarika, P.S. Metallurgical 19,200. Bishnupur, (Ferro-alloys - 00 Dist. Bankura 57600 MTA) 7 Rohit Ferro Tech Pvt. Ltd. PO. Dwarika, P.S. Metallurgical 12,144. (Unit-II) Bishnupur, (Ferro-alloys - 00 Dist. Bankura, PIN. 722122 18000 MTA) Metallurgical 14,628. (Ferro-alloys - 00 12192MTA) DIST. Burdwan 1 Alloy Steel Plant Durgapur, Dist. Burdwan Metallurgical (Alloy Steel- 208149 MTA) 2 Alstom Projects Durgapur, Dist. Burdwan Engineering 0.20 India Ltd. (Steel (formerly Alstom Fabrication - Power Boilers Ltd.) 1500 MTA) 3 Associated Trade G. T. Road, PO. Ningha, PS. Oxyzen gas Link Jamuria, Asansol, PIN. filling & storing 713370 centre Dist. -

(Plantation) Total : 40.02 112.13 Tea Board of India Licensing Department
Tea Board of India Licensing Department List of New Registration of Tea Estate during :from 01/01/1930 To 02/05/2008 Date: 02/05/2008 Page 1 of 121 Registration Date Name of the Company Area Applied Grant Area Mode of Leaf File No No Tea -Estate Processing Revenue District : AGARTALA Sub Division : AGARTALA Plantation Dist : AGARTALA 2659 14/12/1979 Tachi Tea Estate TEA PLANTATION CO-OPERATIVE SOCIETY LTD. 40.02 112.13 NC/PART-361/LC (Plantation) Total : 40.02 112.13 Tea Board of India Licensing Department List of New Registration of Tea Estate during :from 01/01/1930 To 02/05/2008 Date: 02/05/2008 Page 2 of 121 Registration Date Name of the Company Area Applied Grant Area Mode of Leaf File No No Tea -Estate Processing Revenue District : AGARTALA Sub Division : AGARTALA Plantation Dist : Tripura 693 01/04/1953 ADARINI TEA ESTATE MR.M.K.CHOWDHURY 65 131.5 A-501/LC 188 01/04/1953 BRAHMAKUNDU TEA ESTATE BRAHMAKUNDU TEA INDUSTRIES (PVT) LTD 75 150.24 B-788/LC 170 18/02/1958 DURGABARI TEA ESTATE (T-2) DURGABARI TEA ESTATE WORKERS CO-OPERATIVE 44.53 68.01 N-8/LC/57(VOL-2) SOCIETY LTD. 135 18/06/1965 GOLOKPUR TEA ESTATE GOLOKPUR TEA CO. LTD 175 420 G-18/LC 116 01/04/1953 HARENDRANAGAR TEA ESTATE BORGANG TEA CO. (P) LTD., 203 264 H-789/LC 2567 20/04/1953 HARIDASPUR TEA ESTATE TRIPURA PRODUCE CO. 21.67 38.45 H-601/LC 308 11/03/1961 LEELAGARH TEA ESTATE LEELAGARH CHA BAGAN SRAMIK SAMABAYA SAMITY 36.43 137.4 L-16/LC LTD. -

Survey Report AVAILABLE SULPHUR STATUS of DOOARS TEA SOILS
Survey Report AVAILABLE SULPHUR STATUS OF DOOARS TEA SOILS J.J. Ghosh*, U. ~eor~e'and N. Barpujari' ABSTRACT Available sulphur was determined on 5077 soils from the tea growing areas of Dooars (North Bengal), received for routine soil testing between 2000-2003. Over 43% of the soils had more than the critical limit of 40 ppm available sulphur. A distinct geographical trend in sulphur availability was observed, with the four western sub-districts showing a greater frequency of high available sulphur than the three eastern sub-districts. As expected, increased organic carbon in the soils could be correlated by a quadratic equation to the available sulphur status. Three sub-districts, Chulsa, Binnaguri and Dalgaon did not show a high correlation between organic carbon and available sulphur. Surprisingly, a negative linear relationship could be observed between soil pH and available sulphur, in all but the Jainti sub-district, This may be compounded by a negative correlation between soil pH and organic carbon status, in all but the Binnaguri, Kalchini and Jainti sub-districts. Keywords: Available sulphur, Camellia sinensis; Dooars; inter-relationships; soil acidity; soil organic carbon; subdistricts; INTRODUCTION monitor the possibility, the Tea Research Association has been testing available Tea is the economic life-blood of North sulphur routinely in all soils sent for testing in Bengal. Approximately 80,000 hectares of its seven soil testing laboratories from May the Dooars region is under this crop, with an 1998. Soil samples from the Dooars are sent average yield of 1800 KMTH (kilograms to the laboratory at the Nagrakata sub- "made tea" per hectare). -
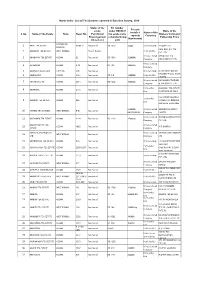
Draft Tea Directory
North India - List of Tea Gardens covered in Baseline Survey, 2018 Status of the RC number Present estate under TMCO (if Name of the installed Status of the S. No Name of the Estate State Regd. No. (Functional/ the estate owns Owners/ Company/ capacity Company Project garden/ a manufacturing Patnership Firms (kgs/annum) Closed etc) unit) ARUNACHAL 1 ABALI TEA ESTATE NEGB-9 Functional RC-1955 50000 Proprietorship JATON PULU PRADESH RAJA ROY, B.R. TEA 2 AARAKAN TEA ESTATE WEST BENGAL Project Garden Public Ltd Co PVT. LTD. Private Limited ABHOYJAN TEA 3 ABHOYJAN TEA ESTATE ASSAM 82 Functional RC-1504 1200000 Company COMPANY PVT LTD Private Limited 4 ACHABAM ASSAM A 58 Functional RC-154 1400000 Company 5 ADARANI TEA ESTATE TRIPURA 693 Functional Proprietorship SUJOY CHOUDHURY MCLEOD RUSSEL INDIA 6 ADDABARIE ASSAM N 24 Functional RC 216 1500000 Public Ltd Co LIMITED Private Limited BHUWALKA TRADING 7 AENAKHALL T.E. ASSAM 2471 Functional RC-1282 1800000 Company & TEA CO.PVT. LTD. Partnership AGARWAL TEA ESTATE 8 AGARWAL ASSAM 2715 Functional firm (PARTNERSHIP FIRM) MOHAN KR. GOENKA, Partnership 9 AHMEDY TEA ESTATE ASSAM 336 Functional MUKESH KR. GOENKA, firm DINDAYAL BAGRODIA 2200000 Private Limited GOODRICKE GROUP 10 AIBHEEL TEA GARDEN WEST BENGAL B-81 Functional KGS/ANNUM Company LIMITED Private Limited THENGALBARI ESTATES 11 AIDEOBARI TEA ESTATE ASSAM 2124 Functional RC-1192 1500000 Company PVT LTD AIDEOPUKHURI TEA Private Limited 12 ASSAM 1065 Functional NITI BANRAH ESTATE Company AIRAN PLANTATION (P) Private Limited AIRAN PLANTATION (P) 13 WEST BENGAL Functional LTD Company LTD 14 AKHOIDESSA TEA ESTATE ASSAM 375 Functional Proprietorship J.N. -
LOK SABHA UNSTARRED QUESTION NO. 3633 (H) to BE ANSWERED on 17Th MARCH, 2021
GOVERNMENT OF INDIA MINISTRY OF COMMERCE & INDUSTRY (DEPARTMENT OF COMMERCE) LOK SABHA UNSTARRED QUESTION NO. 3633 (H) TO BE ANSWERED ON 17th MARCH, 2021 TEA GARDENS 3633 (H). SHRI NABA KUMAR SARANIA: Will the Minister of COMMERCE & INDUSTRY (वािण�य एवं उ�ोग मं�ी) be pleased to state: (a) the total number along with the total area of tea gardens in the country, the details and the names thereof; (b) whether any subsidy is being given by the Government to tea companies and if so, the time by when the daily wages of the labourers are likely to be brought at par with the national parameters as daily wage of the labourers working in tea gardens is Rs.167/-; (c) whether minimum wages does not apply on labourers working in tea gardens and if so, the details thereof; (d) the details of the facilities provided by the company to labourers working in tea gardens; and (e) the details of the profit made by the various tea garden companies during the last three years and the details of the funds spent from CSR fund during thelast five years? ANSWER okf.kT; ,oa m|ksx ea=ky; esa jkT; ea=h ¼Jh gjnhi flag iqjh½ THE MINISTER OF STATE IN THE MINISTRY OF COMMERCE AND INDUSTRY (SHRI HARDEEP SINGH PURI) (a) There are 1569 tea gardens in the country with a total tea area of 420671 Ha. The details of tea gardens with names are given in Annexure-I . (b)& (c): The Central Government through the Tea Board is implementing “Tea Development and Promotion Scheme (TDPS)’’ for development of the Tea Sector. -

STG NL 13.Spub
Chaa Sambad the voice of small tea growers ISSUE 13 | JUNE 2014 Collective Leaf Trade Gives Wrath of Heat Hits Small Tea Growers Windfall Gains to STGs Kaustav Roy, Priyanka Bharadwaj Leaf trade is the stage when small tea March and April depending on the growers directly (avoiding leaf agents) sell region and the age of the tea bush. their leaf to processing factories. Initial feedback of the growers In 2013, members of 69 PPS (about 4500 suggests that the loss may amount STGs) who have been engaged in regular to over 40 per cent of the output. In supply of tea leaves collectively to BLFs/ Golaghat and Jorhat, tea farmers Estate Factories earned as high as Rs 72.19 have experienced output as low as million more than they were earning prior to 5 kg to 6 kg from a bigha as project intervention. compared to over 300 kg per month during these months in the The 69 PPS together handled leaf trade of Rs previous years. In Uttar Dinajpur 180.79 million during 2013. and Jalpaiguri, almost 60 per cent loss in production was reported in the months of March and April 2014 Millions of small tea growers across the country when compared to the same period in 2013. have been affected by a severe draught since “My production in March-April was only 650 kg, the beginning of the plucking season this year. a loss of about 50 per cent,” informed P Rongpi Throughout the month of April, lower rains have from Karbi Anglong District of Assam. -
Social and Cultural History of a Bengal District, Jalpaiguri (1869- 1994)
SOCIAL AND CULTURAL HISTORY OF A BENGAL DISTRICT, JALPAIGURI (1869- 1994) A THESIS SUBMITTED TO THE UNIVERSITY OF NORTH BENGAL FOR THE AWARD OF DOCTOR OF PHILOSOPHY IN HISTORY BY POULAMI BHAWAL GUIDE PROFESSOR ANANDA GOPAL GHOSH (Retired) DEPARTMENT OF HISTORY UNIVERSITY OF NORTH BENGAL 2016 FEBRUARY DECLARATION I declare that the thesis entitled ‘SOCIAL AND CULTURAL HISTORY OF A BENGAL DISTRICT, JALPAIGURI (1869- 1994)’ has been prepared by me under the guidance of Dr. Ananda Gopal Ghosh, Retired Professor, Department of History, University of North Bengal. No part of this thesis has formed the basis for the award of any degree or fellowship previously. Poulami Bhawal Department of History North Bengal University, Raja Rammohanpur District- Darjeeling, West Bengal, Date: 20.02.2016 Abstract Title: Social and Cultural History of a Bengal District, Jalpaiguri (1869-1994) The district Jalpaiguri, in the past, had a discriminative character unlike other parts of Bengal since its inception considering various aspects such as socio-economic-demographic pattern, ethnic societies, geographical location, land revenue purpose and lastly the characteristics of peasants and agrarian relations. Jalpaiguri district was formed in 1869 with the Western Dooars, situated in the Eastern part of Tista and the Sadar Sub-Division comprising with Baikunthapur Pargana, Chaklas of Boda, Patgram and Purbabhag, situated in the Western Part of Tista. It should be noted that on the eve of the formation of the district, the Dooars area was mainly dominated by the tribal people on the hilly areas and the people of Rajbanshi community in the plain areas of the Dooars. The society of the tribal people was mainly ethnic based. -

Survey of Elephant Movement Paths/Corridors Across Rail Tracks in Northern West Bengal
SURVEY OF ELEPHANT MOVEMENT PATHS/CORRIDORS ACROSS RAIL TRACKS IN NORTHERN WEST BENGAL Interim Report to International Elephant Foundation PERIOD OF PROJECT: 16/2/2015–15/02/2016 MUKTI ROY AND RAMAN SUKUMAR Principal Investigator: Dr. Mukti Roy Asian Nature Conservation Foundation C/o Centre for Ecological Sciences, Indian Institute of Science Bangalore 560012 India Co-Investigator: Dr. Raman Sukumar, Centre for Ecological Sciences, Indian Institute of Science Bangalore 560012 India ASIAN NATURE CONSERVATION FOUNDATION C/o CENTRE FOR ECOLOGICAL SCIENCES INDIAN INSTITUTE OF SCIENCE BANGALORE - 560 012, INDIA 1 2. List the overall and specific conservation needs this project addressed 1. Mapping elephant crossing points, and movement paths along the railway line (Siliguri– Alipurduar) passing through forests, tea gardens and agriculture in northern West Bengal, India. 2. Generate susceptibility map showing locations prone to accidents while elephants cross the railway track in order to recommend mitigation measures such as slowing the speed of trains, installing early warning electronic systems or even building elevated tracks for trains or underground passages for elephants across the track. 3. Mapping of elephant movement paths in non-forest areas, especially tea gardens, to inform policy makers about protection of such corridors. 3. Summarize the goals and objectives and describe any changes in goals and objectives from the original proposal There have been no changes so far from the original project proposal. 4. For each objective, describe the specific actions taken to achieve that objective Objective 1:Mapping of elephant crossing points, and movement paths along the railway track (Siliguri–Alipurduar) stretch of northern West Bengal. -

Roadways in Colonial Jalpaiguri District
Roadways in Colonial Jalpaiguri District Debkumar Sengupta Lecture in History,PMS Mahavidyalaya,Jalpaiguri During the colonial rule, i.e. prior independence, there had been significant developments in the roadways in Jalpaiguri. Before the advent of Britishers in Jalpaiguri, there existed but a few roads. The region towards the west of Teesta River, Duars had been a place of great importance as its name is derived from the word ‘Dooar’ which means door-Door to Bhutan. This region served as a gateway to Bhutan at five distinct doors or routes. In short, these routes connected North Bengal to Bhutan. Also in that period in Jalpaiguri researchers have discovered traces of roads (kutcha), one such road name ‘Gosai Kamal’ is still available in Chhipra village near Alipurduar, though those were in a very impoverished state. British officials Sunders and E.E.Lowis have also noted the same facts in their records. In one such report, dated 5th July, 1890 E.E.Lowis, Commissioner of Jalpaiguri stated, ‘the sub division was an outline and remote one, containing but few roads’1. During that time, the whole of Duars region was in a sensitive and unsettled state without proper means of roadway communication. In due course of time, the colonial administration started taking active participation towards development of communication network in Jalpaiguri. The British authority found it a profitable venture in developing this province. In 1884, it was felt by the Deputy Commissioner of Jalpaiguri that the disbursement of fund for the improvement of communication system in the region would be ‘good investment’2, and in this context he suggested taking loans for reproductive public works. -

Elephant Corridors of India
RIGHT OF PASSAGE ELEPHANT CORRIDORS OF INDIA Edited by Vivek Menon, Sandeep Kumar Tiwari, P. S. Easa and R. Sukumar Advisory Board A.J.T.Johnsingh, Anwaruddin Choudhury, Ashok Kumar, J.C.Daniel, K.K.Singh, M.C.Malakar, P.K.Surendranathan Asari, S.C.Mohanty, Srikant Chandola and U.R.Biswas Approved by the State Forest Departments of Arunachal Pradesh, Assam, Jharkhand, Karnataka, Kerala, Meghalaya, Orissa, Tamil Nadu, Uttaranchal and West Bengal Supported by CONSERVATION REFERENCE SERIES NO. 3 The Wildlife Trust of India (WTI) is a non-profit conservation organization committed to initiate and catalyse actions that prevent destruction of India's wildlife and its habitat. In the long run, it aims to achieve, through proactive reforms in policy and manage- ment, an atmosphere conducive to conservation. WTI works through building partner- ships and alliances and its strengths lie in its willingness to work with innovative con- servation techniques like acquiring land for wildlife and rescue and rehabilitation. Suggested Citation: <Author names> 2005. <Paper title> In: Right of Passage: Elephant Corridors of India. Menon, V.,Tiwari, S. K., Easa P. S. and Sukumar, R. (2005). (Eds.). Conservation Reference Series 3. Wildlife Trust of India, New Delhi. Pp Keywords: Conservation, Wild Lands, Assam, Arunachal Pradesh, Jharkhand, Karnataka, Kerala, Meghalaya, Orissa, Tamil Nadu, Uttaranchal, West Bengal, Elephant Corridors, Asian Elephant, Habitat Securement, India. The designations of geographical entities in this publication and the presentation of the material do not imply the expression of any opinion whatsoever on the part of the authors or WTI concerning the legal status of any country, territory or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries All rights reserved.