Bendamustine in Patients with Relapsed Chronic Lymphocytic Leukaemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

WO 2018/190719 A2 18 October 2018 (18.10.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/190719 A2 18 October 2018 (18.10.2018) W !P O PCT (51) International Patent Classification: Not classified (21) International Application Number: PCT/NL20 18/050234 (22) International Filing Date: 13 April 2018 (13.04.2018) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 2018708 13 April 2017 (13.04.2017) NL 2019166 03 July 2017 (03.07.2017) NL (71) Applicant: ADURO BIOTECH HOLDINGS, EUROPE B.V. [NL/NL]; Kloosterstraat 9 RX1 101, 5349 AB Oss (NL). (72) Inventors: VAN EENENNAAM, Hans; c/o Klooster straat 9 RX1 101, 5349 AB Oss (NL). VAN ELSAS, An- drea; c/o Kloosterstraat 9 RX1 101, 5349 AB Oss (NL). = VOETS, Erik; c/o Kloosterstraat 9 RX1 101, 5349 AB Oss = (NL). VINK, Paul; c/o Kloosterstraat 9 RX1 101, 5349 AB = Oss (NL). HULSIK, David Lutje; c/o Kloosterstraat 9 = RX1 101, 5349 AB Oss (NL). = (74) Agent: JANSEN, CM.; V.O., Camegieplein 5, 25 17 KJ = Den Haag (NL). (81) Designated States (unless otherwise indicated, for every = kind of national protection available): AE, AG, AL, AM, = AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, ≡ CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, = DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, ≡ HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, ≡ KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, ≡ MG, MK, MN, MW, MX, MY, MZ, NA, NG, N NO, NZ, = OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, ≡ SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, = TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Hematology Drugs in the Pipeline
102 HemOnc today | NOVEMBER 25, 2015 | Healio.com/HemOnc Hematology Drugs in the Pipeline HEMONC TODAY presents the most recent information about hematology drugs in the pipeline. Drugs listed here are in phase 2 or phase 3 development for a variety of indications. Clinicians can use this chart as a quick reference to learn about the status of those drugs that may be clinically significant to their practice. To view the entire chart online, go to www.Healio.com/HematologyPipeline. Generic name (Brand name, Manufacturer) Indication(s) Development status A6 peptide (Angstrom Pharmaceuticals) chronic lymphocytic leukemia, small lymphocytic lymphoma phase 2 AAV5-hFIX (UniQure Biopharma) hemophilia phase 2 ABC294640 (RedHill Biopharma) diffuse large B-cell lymphoma phase 2 abemaciclib (Eli Lilly) mantle cell lymphoma phase 2 abexinostat (Pharmacyclics) follicular lymphoma, mantle cell lymphoma phase 2 ABP 798 (Allergan/Amgen) non-Hodgkin’s lymphoma phase 3 ACP-196 (Acerta Pharma) B-cell malignancies (combination therapy), mantle cell lymphoma phase 2 READ PERSPECTIVE on this drug from Richard R. chronic lymphocytic leukemia phase 3 Furman, MD, on page 108. ACP-319 (Acerta Pharma) B-cell lymphoma (combination therapy), chronic lymphocytic leukemia phase 2 (combination therapy) adeno-associated virus serotype 8 Factor IX gene therapy hemophilia B phase 2 (AskBio009, Baxalta) AEB071 (Novartis) diffuse large B-cell lymphoma (combination therapy for CD79-mutant or phase 2 ABC-subtype disease) AFM 13 (Affimed Therapeutics) Hodgkin’s lymphoma phase -

Safety, Tolerability, and Preliminary Activity of IMGN529, a CD37
Investigational New Drugs https://doi.org/10.1007/s10637-018-0570-4 PHASE I STUDIES Safety, tolerability, and preliminary activity of IMGN529, a CD37-targeted antibody-drug conjugate, in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: a dose-escalation, phase I study Anastasios Stathis1 & Ian W. Flinn2 & Sumit Madan3 & Kami Maddocks4 & Arnold Freedman5 & Steven Weitman3 & Emanuele Zucca1 & Mihaela C. Munteanu6 & M. Lia Palomba7 Received: 22 January 2018 /Accepted: 6 February 2018 # The Author(s) 2018. This article is an open access publication Summary Background CD37 is expressed on B-cell lymphoid malignancies, thus making it an attractive candidate for targeted therapy in non-Hodgkin lymphoma (NHL). IMGN529 is an antibody-drug conjugate comprising a CD37-binding antibody linked to the maytansinoid DM1, a potent anti-mitotic agent. Methods This first-in-human, phase 1 trial recruited adult patients with relapsed or refractory B-cell NHL. The primary objective was to determine the maximum tolerated dose (MTD) and recommended phase 2 dose. Secondary objectives were to evaluate safety, pharmacokinetics, and preliminary clinical activity. IMGN529 was ad- ministered intravenously once every 3 weeks, and dosed using a conventional 3 + 3 dose-escalation design. Results Forty-nine patients were treated at doses escalating from 0.1 to 1.8 mg/kg. Dose limiting toxicities occurred in eight patients and included peripheral neuropathy, febrile neutropenia, neutropenia, and thrombocytopenia. The most frequent treatment-emergent adverse events were fatigue (39%), neutropenia, pyrexia, and thrombocytopenia (each 37%). Adverse events led to treatment discontin- uation in 10 patients (20%). Eight patients (16%) had treatment-related serious adverse events, the most common being grade 3 febrile neutropenia. -

Leukemia Drugs in the Pipeline
32 HemOnc today | MAY 10, 2016 | Healio.com/HemOnc Leukemia Drugs in the Pipeline HEMONC TODAY presents this guide to drugs in phase 2 or phase 3 development for leukemia. Clinicians can use this chart as a quick reference to learn about the status of those drugs that may be clinically significant to their practice. Generic name (Brand name, Manufacturer) Indication(s) Development status Å6 peptide (Angstrom Pharmaceuticals) chronic lymphocytic leukemia phase 2 acalabrutinib (ACP-196, Acerta Pharma) chronic lymphocytic leukemia (relapsed/refractory, ibrutinib-intolerant disease) phase 2 chronic lymphocytic leukemia (monotherapy for previously treated high-risk phase 3 disease; combination therapy for previously untreated disease) AG-120 (Agios Pharmaceuticals and Celgene) acute myeloid leukemia (combination therapy for newly diagnosed disease) phase 2 AG-221 (Agios Pharmaceuticals and Celgene) acute myeloid leukemia (older patients with IDH2–mutated late-stage phase 3 disease) alvocidib (Tolero Pharmaceuticals) acute myeloid leukemia phase 2 AST-VAC1 (Asterias Biotherapeutics) acute myelogenous leukemia phase 2 Bismab-A2 (Actinium Pharmaceuticals) acute myelogenous leukemia phase 2 BL-8040 (BioLineRx) acute myeloid leukemia phase 2 blinatumomab (Amgen) acute lymphoblastic leukemia (Philadelphia chromosome-positive or BCR- phase 2 ABL–positive disease; patients with B-cell lineage with minimal residual dis- ease; combination therapy for older patients with newly diagnosed disease) acute lymphoblastic leukemia (relapsed disease; pediatric -
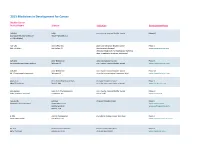
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data.