Insect Odor and Taste Receptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Review Officer Manual
Department of Health and Human Services Substance Abuse and Mental Health Services Administration Center for Substance Abuse Prevention Medical Review Officer Manual for Federal Agency Workplace Drug Testing Programs EFFECTIVE OCTOBER 1, 2010 Note: This manual applies to Federal agency drug testing programs that come under Executive Order 12564 dated September 15, 1986, section 503 of Public Law 100-71, 5 U.S.C. section 7301 note dated July 11, 1987, and the Department of Health and Human Services Mandatory Guidelines for Federal Workplace Drug Testing Programs (73 FR 71858) dated November 25, 2008 (effective October 1, 2010). This manual does not apply to specimens submitted for testing under U.S. Department of Transportation (DOT) Procedures for Transportation Workplace Drug and Alcohol Testing Programs (49 CFR Part 40). The current version of this manual and other information including MRO Case Studies are available on the Drug Testing page under Medical Review Officer (MRO) Resources on the SAMHSA website: http://www.workplace.samhsa.gov Previous Versions of this Manual are Obsolete 3 Table of Contents Chapter 1. The Medical Review Officer (MRO)........................................................................... 6 Chapter 2. The Federal Drug Testing Custody and Control Form ................................................ 7 Chapter 3. Urine Drug Testing ...................................................................................................... 9 A. Federal Workplace Drug Testing Overview.................................................................. -

Functional Properties of Insect Olfactory Receptors: Ionotropic Receptors and Odorant Receptors
Cell and Tissue Research (2021) 383:7–19 https://doi.org/10.1007/s00441-020-03363-x REVIEW Functional properties of insect olfactory receptors: ionotropic receptors and odorant receptors Dieter Wicher1 · Fabio Miazzi2 Received: 14 August 2020 / Accepted: 19 November 2020 / Published online: 27 January 2021 © The Author(s) 2021 Abstract The majority of insect olfactory receptors belong to two distinct protein families, the ionotropic receptors (IRs), which are related to the ionotropic glutamate receptor family, and the odorant receptors (ORs), which evolved from the gustatory recep- tor family. Both receptor types assemble to heteromeric ligand-gated cation channels composed of odor-specifc receptor proteins and co-receptor proteins. We here present in short the current view on evolution, function, and regulation of IRs and ORs. Special attention is given on how their functional properties can meet the environmental and ecological challenges an insect has to face. Keywords Insect olfaction · Ionotropic receptor · Odorant receptor · Ion channel · Olfactory sensory neuron · Signal transduction · Sensitization · Adaptation Introduction receptors (IRs). The frst members of the OR family were discovered two decades ago (Clyne et al. 1999; Gao and The olfactory system is dedicated to detect and to encode Chess 1999; Vosshall et al. 1999), whereas the IRs that are information from volatile chemical signals. Such signals can related to ionotropic glutamate receptors were frst reported be categorized according to the information they transfer. ten years later (Benton et al. 2009). For example, chemosignals involved in social communica- While ORs solely detect volatile chemosignals, IRs tion may be informative solely for the receiver as an olfac- are multimodal receptive entities (Fig. -

Color, Taste, and Odor: What You Should Know
Color, Taste, and Odor: What you should know From time to time the MassDEP receives consumer questions or complaints regarding the look, taste or the odor of drinking water. Listed below are common problems with drinking water and their most common causes. Please note that a particular problem in your drinking water may be the result of a cause not listed here; the only way to confirm a cause is to have a certified lab analyze the water and discuss the results with drinking water professional. If you receive water from a public drinking water system it is important to contact the Public Water Supply (PWS) before having a laboratory analyze the water. Information on private water testing is available. Filtering or treating the water may remedy persistent problems; however MassDEP does not recommend filtering or treating your water supply if your water is supplied by a MassDEP- approved PWS. MassDEP also does not regulate or recommend specific treatment systems for private home use. If you decide to use a filtration or treatment device in your home, the Department strongly encourages you to contact National Sanitation Foundation (NSF) for a list of approved devices. If you purchase a treatment device for private home use MassDEP also strongly recommends that it is maintained and provide active maintenance according to the manufacturer's instructions. Failure to maintain the equipment properly may make treatment ineffective and/or may create the potential for contamination. Common problems with drinking water are grouped into three categories: Color problems Taste / odor problems Particles in water If the problem with your water is not described here, if you are on a public water system please contact the public water department in your city or town or the MassDEP Drinking Water Program at your nearest regional MassDEP office. -

Altered Functional Properties of the Codling Moth Orco Mutagenized in the Intracellular Loop‑3 Yuriy V
www.nature.com/scientificreports OPEN Altered functional properties of the codling moth Orco mutagenized in the intracellular loop‑3 Yuriy V. Bobkov1, William B. Walker III2 & Alberto Maria Cattaneo1,2* Amino acid substitutions within the conserved polypeptide sequence of the insect olfactory receptor co‑receptor (Orco) have been demonstrated to infuence its pharmacological properties. By sequence analysis and phylogenetic investigation, in the Lepidopteran subgroup Ditrysia we identifed a fxed substitution in the intracellular loop‑3 (ICL‑3) of a conserved histidine to glutamine. By means of HEK293 cells as a heterologous system, we functionally expressed Orco from the Ditrysian model Cydia pomonella (CpomOrco) and compared its functional properties with a site‑directed mutagenized version where this ICL‑3‑glutamine was reverted to histidine (CpomOrcoQ417H). The mutagenized CpomOrcoQ417H displayed decreased responsiveness to VUAA1 and reduced response efcacy to an odorant agonist was observed, when co‑transfected with the respective OR subunit. Evidence of reduced responsiveness and sensitivity to ligands for the mutagenized Orco suggest the fxed glutamine substitution to be optimized for functionality of the cation channel within Ditrysia. In addition, contrary to the wild type, the mutagenized CpomOrcoQ417H preserved characteristics of VUAA‑binding when physiologic conditions turned to acidic. Taken together, our fndings provide further evidence of the importance of ICL‑3 in forming basic functional properties of insect Orco‑ and Orco/OR‑channels, and suggest involvement of ICL‑3 in the potential functional adaptation of Ditrysian Orcos to acidifed extra‑/intracellular environment. Te odorant receptor co-receptor, Orco, is a unique transmembrane protein, expressed in most of the olfac- tory sensory neurons (OSNs) of insect antennae1–3 and is highly conserved in sequence and function across all insects4,5. -
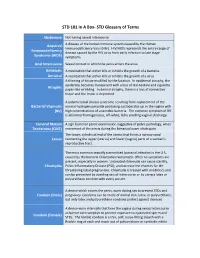
STD Glossary of Terms
STD 101 In A Box- STD Glossary of Terms Abstinence Not having sexual intercourse Acquired A disease of the human immune system caused by the Human Immunodeficiency Virus (HIV). HIV/AIDS represents the entire range of Immunodeficiency disease caused by the HIV virus from early infection to late stage Syndrome (AIDS) symptoms. Anal Intercourse Sexual contact in which the penis enters the anus. Antibiotic A medication that either kills or inhibits the growth of a bacteria. Antiviral A medication that either kills or inhibits the growth of a virus. A thinning of tissue modified by the location. In epidermal atrophy, the epidermis becomes transparent with a loss of skin texture and cigarette Atrophic paper-like wrinkling. In dermal atrophy, there is a loss of connective tissue and the lesion is depressed. A polymicrobial clinical syndrome resulting from replacement of the Bacterial Vaginosis normal hydrogen peroxide producing Lactobacillus sp. in the vagina with (BV) high concentrations of anaerobic bacteria. The common symptom of BV is abnormal homogeneous, off-white, fishy smelling vaginal discharge. Cervical Motion A sign found on pelvic examination suggestive of pelvic pathology; when Tenderness (CMT) movement of the cervix during the bimanual exam elicits pain. The lower, cylindrical end of the uterus that forms a narrow canal Cervix connecting the upper (uterus) and lower (vagina) parts of a woman's reproductive tract. The most common sexually transmitted bacterial infection in the U.S., caused by the bacteria Chlamydia trachomatis. Often no symptoms are present, especially in women. Untreated chlamydia can cause sterility, Chlamydia Pelvic Inflammatory Disease (PID), and increase the chances for life- threatening tubal pregnancies. -

A Pheromone Antagonist Liberates Female Sea Lamprey from a Sensory Trap to Enable Reliable Communication
A pheromone antagonist liberates female sea lamprey from a sensory trap to enable reliable communication Tyler J. Buchingera,1, Anne M. Scotta,1, Skye D. Fissettea, Cory O. Branta,2, Mar Huertasa,3,KeLia,4, Nicholas S. Johnsonb, and Weiming Lia,5 aDepartment of Fisheries and Wildlife, Michigan State University, East Lansing, MI 48824; and bHammond Bay Biological Station, US Geological Survey, Millersburg, MI 49759 Edited by John G. Hildebrand, University of Arizona, Tucson, AZ, and approved February 21, 2020 (received for review December 12, 2019) The evolution of male signals and female preferences remains a subsequent preference evolution precludes a full understanding central question in the study of animal communication. The sensory of how receiver biases shape communication. trap model suggests males evolve signals that mimic cues used Sea lamprey (Petromyzon marinus) are a useful model to track in nonsexual contexts and thus manipulate female behavior to the evolutionary trajectory of communication that originated via generate mating opportunities. Much evidence supports the sen- a sensory trap. Chemical cues and pheromones guide sea lam- sory trap model, but how females glean reliable information from prey behavior throughout their complex life history, especially both mimetic signals and their model cues remains unknown. We during the terminal reproductive phase (21). After parasitizing discovered a mechanism whereby a manipulative male signal guides fish in lakes or the Atlantic Ocean, prespawning sea lamprey reliable communication in sea lamprey (Petromyzon marinus). Mi- migrate into streams following chemical cues released by larvae gratory sea lamprey follow a larval cue into spawning streams; once residing in nursery habitats near spawning grounds. -

Visions & Reflections on the Origin of Smell: Odorant Receptors in Insects
Cell. Mol. Life Sci. 63 (2006) 1579–1585 1420-682X/06/141579-7 DOI 10.1007/s00018-006-6130-7 Cellular and Molecular Life Sciences © Birkhäuser Verlag, Basel, 2006 Visions & Reflections On the ORigin of smell: odorant receptors in insects R. Benton Laboratory of Neurogenetics and Behavior, The Rockefeller University, 1230 York Avenue, Box 63, New York, New York 10021 (USA), Fax: +1 212 327 7238, e-mail: [email protected] Received 23 March 2006; accepted 28 April 2006 Online First 19 June 2006 Abstract. Olfaction, the sense of smell, depends on large, suggested that odours are perceived by a conserved mecha- divergent families of odorant receptors that detect odour nism. Here I review recent revelations of significant struc- stimuli in the nose and transform them into patterns of neu- tural and functional differences between the Drosophila ronal activity that are recognised in the brain. The olfactory and mammalian odorant receptor proteins and discuss the circuits in mammals and insects display striking similarities implications for our understanding of the evolutionary and in their sensory physiology and neuroanatomy, which has molecular biology of the insect odorant receptors. Keywords. Olfaction, odorant receptor, signal transduction, GPCR, neuron, insect, mammal, evolution. Olfaction: the basics characterised by the presence of seven membrane-span- ning segments with an extracellular N terminus. OR pro- Olfaction is used by most animals to extract vital infor- teins are exposed to odours on the ciliated endings of olf- mation from volatile chemicals in the environment, such actory sensory neuron (OSN) dendrites in the olfactory as the presence of food or predators. -

Taste and Odor Control, but Use As a Control Chemical Must Be Evaluated Carefully Due to the Formation of Thms and Chlorophenol When Organics Are Present
Taste and Odor Most customers judge the quality of drinking water by taste and odor. If the customer is satisfied with these qualities, it is assumed the water is safe to drink. Many harmful contaminants in water cannot be detected due to taste or smell and many of the contaminants found in drinking water that have a detectable taste or odor are not harmful. Sources of taste and odor problems can be found in surface water and groundwater. Source water protection involves the prevention of contaminants from entering the source. Surface water or groundwater may become contaminated by pollutants such as gasoline, industrial solvents or a wide variety of volatile organics. The removal of contaminants from surface water or groundwater is costly and may involve the use of aeration, powdered activated carbon, or both. If taste and odor must be controlled at the treatment plant, oxidation, aeration and adsorption can be effective in reducing taste and odor, and improved coagulation filtration. ODOR MEASUREMENTS One of the most common methods for measuring odor in water is the threshold odor test. It involves a series of flasks presented to an observer, who is told that some of the samples contain odors and that the series is arranged in order of increasing concentrations. The observer is also given a known odor-free blank for reference during the test. The observer compares the flasks in ascending order with the blank and then notes whether an odor is detected in any sample flask. Individuals vary in their reactions to certain types of odors. An odor stimulus that is agreeable to one may be disagreeable to another. -

The Role of Chemoreceptor Evolution in Behavioral Change Cande, Prud’Homme and Gompel 153
Available online at www.sciencedirect.com Smells like evolution: the role of chemoreceptor evolution in behavioral change Jessica Cande, Benjamin Prud’homme and Nicolas Gompel In contrast to physiology and morphology, our understanding success. How an organism interacts with its environment of how behaviors evolve is limited.This is a challenging task, as can be divided into three parts: first, the sensory percep- it involves the identification of both the underlying genetic tion of diverse auditory, visual, tactile, chemosensory or basis and the resultant physiological changes that lead to other cues; second, the processing of this information by behavioral divergence. In this review, we focus on the central nervous system (CNS), leading to a repres- chemosensory systems, mostly in Drosophila, as they are one entation of the sensory signal; and third, a behavioral of the best-characterized components of the nervous system response. Thus, behaviors could evolve either through in model organisms, and evolve rapidly between species. We changes in the peripheral nervous system (PNS) (e.g. examine the hypothesis that changes at the level of [1 ]), or through changes in higher-order neural circuitry chemosensory systems contribute to the diversification of (Figure 1). While the latter remain elusive, recent work behaviors. In particular, we review recent progress in on chemosensation in insects illustrates how the PNS understanding how genetic changes between species affect shapes behavioral evolution. chemosensory systems and translate into divergent behaviors. A major evolutionary trend is the rapid Chemosensation in insects depends on three classes of diversification of the chemoreceptor repertoire among receptors expressed in peripheral neurons housed in species. -

Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae Kenta Asahina
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2008 Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae Kenta Asahina Follow this and additional works at: http://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons Recommended Citation Asahina, Kenta, "Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae" (2008). Student Theses and Dissertations. Paper 196. This Thesis is brought to you for free and open access by Digital Commons @ RU. It has been accepted for inclusion in Student Theses and Dissertations by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. Cracking the Odor Code: Molecular and Cellular Deconstruction of the Olfactory Circuit of Drosophila Larvae A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy By Kenta Asahina June 2008 © Copyright by Kenta Asahina 2008 CRACKING THE ODOR CODE: MOLECULAR AND CELLULAR DECONSTRUCTION OF THE OLFACTORY CIRCUIT OF DROSOPHILA LARVAE Kenta Asahina, Ph.D. The Rockefeller University 2008 The Drosophila larva offers a powerful model system to investigate the general principles by which the olfactory system processes behaviorally relevant sensory stimuli. The numerically reduced larval olfactory system relieves the formidable molecular and cellular complexity found in other organisms. This thesis presents a study in four parts that investigates molecular and neuronal mechanisms of larval odor coding. First, the larval odorant receptor (OR) repertoire was characterized. ORs define the olfactory receptive range of an animal. -

Tuning Insect Odorant Receptors
PERSPECTIVE published: 05 April 2018 doi: 10.3389/fncel.2018.00094 Tuning Insect Odorant Receptors Dieter Wicher* Department of Evolutionary Neuroethology, Max Planck Institute for Chemical Ecology (MPG), Jena, Germany Among the insect olfactory receptors the odorant receptors (ORs) evolved in parallel to the onset of insect flight. A special property of this receptor type is the capability to adjust sensitivity of odor detection according to previous odor contacts. This article presents a current view on regulatory processes affecting the performance of ORs and proposes a model of mechanisms contributing to OR sensitization. Keywords: chemoreception, olfaction, ionotropic receptor, odorant receptor, receptor kinase, GPCR, intracellular signaling INTRODUCTION The performance of membrane proteins such as ion channels or receptors is dynamically adjusted according to changing physiological requirements. Olfactory receptors have to detect odors in a wide range of concentrations, from faint filaments at larger distance from the source to high concentrated and permanent presence near the source. In mammals, the olfactory receptors for general odors are G protein coupled receptors (GPCRs; Buck and Axel, 1991). For a comparison of vertebrate and insect olfaction see Kaupp(2010), for a recent review of insect olfactory receptors see Fleischer et al.(2018). Three types of receptor proteins detect volatile chemical information in insects. These are odorant receptors (ORs) which are restricted to insects, specific gustatory receptors (GRs) detecting carbon dioxide and receptors related to ionotropic glutamate receptors, called ionotropic receptors (IRs). The ORs evolved in parallel with the onset of insect flight (Missbach et al., 2014). Similar to GPCRs, insect ORs belong to the class of heptahelical transmembrane proteins. -

Distinct Signaling of Drosophila Chemoreceptors in Olfactory
Distinct signaling of Drosophila chemoreceptors in PNAS PLUS olfactory sensory neurons Li-Hui Caoa,b,c,d, Bi-Yang Jinga,b,c,d,1, Dong Yange,1, Xiankun Zengf,1, Ying Shene, Yuhai Tug, and Dong-Gen Luoa,b,c,d,2 aState Key Laboratory of Membrane Biology, College of Life Sciences, Peking University, Beijing 100871, China; bCenter for Quantitative Biology, Peking University, Beijing 100871, China; cMcGovern Institute for Brain Research, Peking University, Beijing 100871, China; dPeking–Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China; eDepartment of Neurobiology, Zhejiang University School of Medicine, Hangzhou 310058, China; fJanelia Research Campus, Howard Hughes Medical Institute, Ashburn, VA 20147; and gIBM T. J. Watson Research Center, Yorktown Heights, NY 10598 Edited by Richard W. Aldrich, The University of Texas at Austin, Austin, TX, and approved December 24, 2015 (received for review September 15, 2015) In Drosophila, olfactory sensory neurons (OSNs) rely primarily on recordings of single OSNs could ideally overcome this issue while two types of chemoreceptors, odorant receptors (Ors) and iono- facilitating the experimental manipulations of a cell’s membrane tropic receptors (Irs), to convert odor stimuli into neural activity. potential; however, this standard method has unfortunately not The cellular signaling of these receptors in their native OSNs re- yet been routinely applied to Drosophila OSNs. mains unclear because of the difficulty of obtaining intracellular Here, we developed a Drosophila antennal preparation and Drosophila recordings from OSNs. Here, we developed an antennal succeeded in performing patch-clamp recordings of single iden- preparation that enabled the first recordings (to our knowledge) tified OSNs.