Coosa County
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

STREAK Streak Means the Color of a Mineral in Its Powdered Form. The
STREAK Streak means the color of a mineral in its powdered form. The name comes from the common method of observing it by scraping the mineral on a tile of white, unglazed porcelain to produce a narrow streak of powder. Streak is a valuable property because its color is usually the same even when larger samples of many minerals show a variety of colors. Streak is often better for mineral identification than color. Streak of metallic minerals Streak is valuable for identification of many metallic minerals, including native metals (such as gold), oxides and sulfide. Almost all of these minerals have metallic or submetallic luster and are opaque. Their streak is often quite different from their color in a hand sample, generally darker. Examples: Hematite: Hematite can occur as black crystals, steel gray in the form known as specularite, and as dull red earthy masses. Its streak, however, is always blood red. By this simple test hematite can be known apart from other black, metallic minerals of similar appearance. Pyrite: Pyrite comes in a brassy yellow color, but has a black or greenish-black streak. It is sometimes called “fool’s gold” because people mistook specks in ores for real gold. But native gold has a gold streak. Chalcopyrite and marcasite are closely related to pyrite. They have paler brassy colors than pyrite, but also show dark streaks. Bornite: Bornite is bronze color when fresh, but quickly tarnishes to blue and purple shades, and eventually turns black. Its streak, however, is always grayish black. Streak of nonmetallic minerals In contrast, most minerals with nonmetallic lusters are transparent or translucent, and have a streak paler than their color in hand-samples. -

Chapter 3 Nonsilicate Minerals the 8 Most Common Elements in the Continental Crust Elements That Comprise Most Minerals Classification of Minerals
Chapter 3 Nonsilicate Minerals The 8 Most Common Elements in the Continental Crust Elements That Comprise Most Minerals Classification of Minerals ! "Important nonsilicate minerals •" Several major groups exist including –"Oxides –"Sulfides –"Sulfates –"Native Elements –"Carbonates –"Halides –"Phosphates Classification of Minerals ! "Important nonsilicate minerals •" Many nonsilicate minerals have economic value •" Examples –"Hematite (oxide mined for iron ore) –"Halite (halide mined for salt) –"Sphalerite (sulfide mined for zinc ore) –"Native Copper (native element mined for copper) Table 3.2 (Modified) Common Nonsilicate Mineral Groups Classification of Minerals ! "Important nonsilicate minerals •" Oxides – natural compounds in which Oxygen anions combine w/ a variety of metal cations –"Ores of various metals –"Hematite & Magnetite (iron oxides), Chromite (iron-chromium oxide), Ilmenite (iron-titanium oxide) and Ice (H2O – the solid form of water) are some important oxides Hematite (Fe2O3) Ore of Iron Red Streak Metallic to Earthy Luster Black or Red Color Magnetite (Fe3O4) Ore of Iron Strong Magnetism Black Color Hardness (6) Metallic Luster Corundum (Ruby& Sapphire) (Al2O3) Gemstone Hardness = 9 (Scratches Knife), Hexagonal Crystals, Adamantine Luster, Color Variable, But Can Be Red (Ruby) or Blue (Sapphire) Chromite (FeCr2O4) Ore of Chromium Metallic to Submetallic Luster Iron Black to Brownish Black Color Octahedral Crystal Form Hardness (5.5-6) Ilmenite (FeTiO3) Ore of Titanium Black-Brownish Black Streak Metallic to Submetallic -

Mineral Identification
Mineral Identification Let’s identify some minerals! What do good scientists do? GOOD SCIENTISTS… ⚫ work together and share their information. ⚫ observe carefully. ⚫ record their observations. ⚫ learn from their observations and continue to test, examine, and discuss. What are minerals? MINERALS ARE… ⚫ matter. ⚫ inorganic (nonliving) solids found in nature. ⚫ made up of elements such as silicon, oxygen, carbon, iron. ( Si O C Fe) ⚫ NOT ROCKS!! Rocks are made up of minerals. Physical Properties ⚫ What are physical properties of matter? ⚫ Shape, color, texture, size, etc. ⚫ The physical properties of minerals are… ⚫Transparency ⚫Luster ⚫Fracture ⚫COLOR ⚫Cleavage ⚫STREAK ⚫Specific Gravity ⚫Crystal Form ⚫HARDNESS Physical Properties ⚫ Some physical properties of minerals we won’t be examining in this lab: How well light passes ⚫Transparency through ⚫ Fracture/Cleavage How it breaks ⚫ Crystal Form The pattern of its crystal formation Color Some minerals are easy to identify by color. ⚫ Malachite is always green. But the color of other minerals change when there are traces of other elements. ⚫ Quartz is clear in its purest form. ⚫ With traces of iron, it becomes purple…amethyst. ⚫ With traces of manganese, it become pink…rose quartz. Takeaway: Visual color helps in identification. Because visual color can be affected by traces of elements, scientists use another test, of streak color. Streak ⚫ Rubbing a mineral firmly across an unglazed porcelain tile (streak plate) leaves a line of powder. ⚫ The streak color of a mineral is always the same, regardless of its visual color. ⚫ For instance, both amethyst and rose quartz leave the white streak of quartz in its purest form. Takeaway: Streak color is an even more reliable way to identify a mineral. -

Global Building Blocks: Rocks and Mineral Identification
Cave of the Winds Activity Eleven: Global Building Blocks Lesson for Grades 9-12 One lab of about 50 minutes Global Building Blocks: Satisfies Colorado Rocks and Mineral Identification Model Content Standard for Science, Standard 4, Objectives Benchmark #1 for grades The learner will: 9-12: The earth’s interior has 1. Learn about mineral composition and identification. a composition and a structure. 2. Understand the rock cycle and identify some common rocks in each group. Prerequisite: knowledge of Vocabulary elements Mineral, rock, crystal structure, chemical composition, hardness, luster, cleavage, carbonates, rock cycle, igneous rock, metamorphic rock, sediment, clast, chemical sedimentary rock, bio- chemical sedimentary rock, siliciclastic sedimentary rock, sedimentary rock, parent rock, metamorphism, magma, crystallization, cementation, evaporite, metallic, nonmetallic, stria- tions, rhombus, transparent, translucent, physical weathering, chemical weathering, erosion, rock cycle, basalt, granite, trona, limestone, conglomerate, sandstone, shale, coquina, coal, gneiss, schist, marble, slate, mafic, felsic, foliated, crystalline, texture, vesicular, ooids, pebbles, sand, gravel, grain size, clay, organic material, ultraviolet light, hydrochloric acid, efferves- cence, streak, calcite, dolomite, gypsum, halite, sulfur, pyrite, muscovite mica, orthoclase feldspar, quartz. Background Information The Earth’s building blocks are rocks and minerals. Minerals, of which rocks are composed, are defined by standard criteria: 1) specific chemical -

Mineral Collecting Sites in North Carolina by W
.'.' .., Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie RUTILE GUMMITE IN GARNET RUBY CORUNDUM GOLD TORBERNITE GARNET IN MICA ANATASE RUTILE AJTUNITE AND TORBERNITE THULITE AND PYRITE MONAZITE EMERALD CUPRITE SMOKY QUARTZ ZIRCON TORBERNITE ~/ UBRAR'l USE ONLV ,~O NOT REMOVE. fROM LIBRARY N. C. GEOLOGICAL SUHVEY Information Circular 24 Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie Raleigh 1978 Second Printing 1980. Additional copies of this publication may be obtained from: North CarOlina Department of Natural Resources and Community Development Geological Survey Section P. O. Box 27687 ~ Raleigh. N. C. 27611 1823 --~- GEOLOGICAL SURVEY SECTION The Geological Survey Section shall, by law"...make such exami nation, survey, and mapping of the geology, mineralogy, and topo graphy of the state, including their industrial and economic utilization as it may consider necessary." In carrying out its duties under this law, the section promotes the wise conservation and use of mineral resources by industry, commerce, agriculture, and other governmental agencies for the general welfare of the citizens of North Carolina. The Section conducts a number of basic and applied research projects in environmental resource planning, mineral resource explora tion, mineral statistics, and systematic geologic mapping. Services constitute a major portion ofthe Sections's activities and include identi fying rock and mineral samples submitted by the citizens of the state and providing consulting services and specially prepared reports to other agencies that require geological information. The Geological Survey Section publishes results of research in a series of Bulletins, Economic Papers, Information Circulars, Educa tional Series, Geologic Maps, and Special Publications. -

Lab 1 - Physical Properties of Minerals
Page - Lab 1 - Physical Properties of Minerals All rocks are composed of one or more minerals. In order to be able to identify rocks you have to be able to recognize those key minerals that make of the bulk of rocks. By definition, any substance is classified as a mineral if it meets all 5 of the following criteria: - is naturally occurring (ie. not man-made); - solid (not liquid or gaseous); - inorganic (not living and never was alive); - crystalline (has an orderly, repetitive atomic structure); - a definite chemical composition (you can write a discrete chemical formula for any mineral). Identifying an unknown mineral is like identifying any group of unknowns (leaves, flowers, bugs... etc.) You begin with a box, or a pile, of unknown minerals and try to find any group features in the samples that will allow you to separate them into smaller and smaller piles, until you are down to a single mineral and a unique name. For minerals, these group features are called physical properties. Physical properties are any features that you can use your 5 senses (see, hear, feel, taste or smell) to aid in identifying an unknown mineral. Mineral physical properties are generally organized in a mineral key and the proper use of this key will allow you to name your unknown mineral sample. The major physical properties will be discussed briefly below in the order in which they are used to identify an unknown mineral sample. Luster Luster is the way that a mineral reflects light. There are two major types of luster; metallic and non-metallic luster. -

Feruvite, a New Member of the Tourmaline Group, and Its
Canadion Mineralogist Yol.27, pp. 199-203(1989) FERUVITE,A NEW MEMBEROF THE TOURMALINEGROUP, AND ITS CRYSTALSTRUCTURE JOEL D. GRICE AND GEORGE W. ROBINSON Mineral SciencesDivision, National Museumof Natural Scienca,Ottawa, Ontario KIP 6P4 AssrRAC"r i (Cae.62Naj3slq;or)rr.rzGe?j3Mg1.21Tie.2elvlno.or)e:.on (Ala.7 2Mgs.s2Fere-3aFe'ei )s6.00(BO3)3 (Si5. gAlo. ro) Feruvite, ideally CaFq(A1,Mg)e@O:)lSieOls(OH)a,is a rs.srOrr(OH)a.D6"3.21, D-*3.207(9) g/cm3.La struc- new tourmaline species that is associated with quartz, ture, qui estcelle de$ membres de la famille de la tourma- microcline, chlorapatite and pyrite ir a tourmalinized rock line,a €t6affind jusqu'l un r6sidu.R de l.6Vo.la r6parti- at Cuvier Island, New Zealand. Feruvite forms dark brown- tion descations des sites fet Z a aussi&6 affin€e(r€sultats black, equant grains up to 2 mm in diameter. The mineral citdsci-haut). Cette espOce est rare i causede. sa parage- is briille, H about 7, conchoidal fracture, optically uniax- nbseunique. Son nom indiquequ'elle est l'€quivalent fer- ial negativewi& ar1.687(l), e 1.669(l). [t is trigonal, space rifdre de I'uvite (ou l'6quivalentcalcique du schorl). group .R3rn, a 16.012{2),c 7.245(4 A. ard Z=3. TlLe stroqgest X-ray-diffraction lines in the powder pattern [d (Iraduit par la R€daction) in A (, (hkt)l are: 6.43(40xl0l), 4.24(60)QrD, 4.00(60)(220), 3.50(60X012), 2.979(80)(t22), Mots-cl&:feruvite, nouvelle esplce min{mls, groupede la 2.586(l00X0s1), 2.05r(s0x152), r.928(40)(342), tourmaline,lle deCuvier, Nouvelle-Zdlande, affinernent 1.600(40X550)and 1,439(N)Q40).Results from an elechon- de la structure,tourmalinisation. -

Methods Used to Identifying Minerals
METHODS USED TO IDENTIFYING MINERALS More than 4,000 minerals are known to man, and they are identified by their physical and chemical properties. The physical properties of minerals are determined by the atomic structure and crystal chemistry of the minerals. The most common physical properties are crystal form, color, hardness, cleavage, and specific gravity. CRYSTALS One of the best ways to identify a mineral is by examining its crystal form (external shape). A crystal is defined as a homogenous solid possessing a three-dimensional internal order defined by the lattice structure. Crystals developed under favorable conditions often exhibit characteristic geometric forms (which are outward expressions of the internal arrangements of atoms), crystal class, and cleavage. Large, well- developed crystals are not common because of unfavorable growth conditions, but small crystals recognizable with a hand lens or microscope are common. Minerals that show no external crystal form but possess an internal crystalline structure are said to be massive. A few minerals, such as limonite and opal, have no orderly arrangement of atoms and are said to be amorphous. Crystals are divided into six major classes based on their geometric form: isometric, tetragonal, hexagonal, orthorhombic, monoclinic, and triclinic. The hexagonal system also has a rhombohedral subdivision, which applies mainly to carbonates. CLEAVAGE AND FRACTURE After minerals are formed, they have a tendency to split or break along definite planes of weakness. This property is called cleavage. These planes of weakness are closely related to the internal structure of the mineral, and are usually, but not always, parallel to crystal faces or possible crystal faces. -
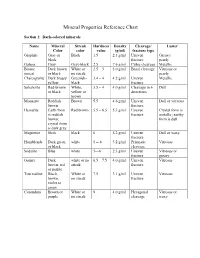
Mineral Properties Reference Chart
Mineral Properties Reference Chart Section 1: Dark-colored minerals Name Mineral Streak Hardness Density Cleavage/ Luster Color color value (g/ml) fracture type Graphite Gray or Black 1.5 2.3 g/ml Uneven Greasy/ black fracture pearly Galena Gray Gray-black 2.5 7.6 g/ml Cubic cleavage Metallic Biotite Dark brown White or 2.5 – 3 3.0 g/ml Basal cleavage Vitreous or (mica) or black no streak pearly Chalcopyrite Dark brassy Greenish- 3.4 – 4 4.2 g/ml Uneven Metallic yellow black fracture Sphalerite Red-brown White, 3.5 – 4 4.0 g/ml Cleavage in 6 Dull or black yellow or directions brown Monazite Reddish Brown 5.5 4.6 g/ml Uneven Dull or vitreous brown fracture Hematite Earth form Red brown 5.5 – 6.5 5.3 g/ml Uneven Crystal form is is reddish fracture metallic; earthy brown; form is dull crystal form is dark gray Magnetite black black 6 5.2 g/ml Uneven Dull or waxy fracture Hornblende Dark green white 5 -- 6 3.2 g/ml Prismatic Vitreous or black cleavage Sodalite Blue white 5—6 2.3 g/ml Uneven Vitreous or fracture greasy Garnet Dark white or no 6.5 – 7.5 4.0 g/ml Uneven Vitreous brown, red streak fracture or purple Tourmaline Black, White or 7.5 3.1 g/ml Uneven Vitreous brown, no streak fracture violet or green Corundum Brown or White or 9 4.0 g/ml Hexagonal Vitreous or purple no streak cleavage waxy Section 2: Light-colored minerals Name Mineral Streak Hardness Density Cleavage/ Luster Color color value (g/ml) fracture type Talc White White 1 2.7 g/ml Basal cleavage Pearly OR uneven fracture Gypsum White White or 2 2.3 g/ml Cleavage in Vitreous, -

100 a Large Cleavage Piece of Pale Pink Bustamite from Franklin
100 TH E AM ERICAN M I N ERA INGI ST A large cleavage piece of pale pink bustamite from Franklin Furnace kindly presented to the U. S. National Museum by Mr. R. B. Gage of Trenton, N. J., has the following optical properties: Optically-, 2V medium. a:1.667, P:1.678,ry: 1-680. Fragments lying on the most perfect cleavageshow the emergence of X out of the field and give variable but small extinction angles. Several specimens from Franklin Furnace that superficially resembledthe bustamite were examinedmicroscopically and proved to have optical properties much like those of the fowlerite variety of rhodonite, though with somewhat lower indices of refraction. Coxcriusrox: The bustamite from Franklin Furnace is a triclinic pyroxene, related to rhodonite. Chemically it contains nearly equal molecular proportions of CaO and MnO with CaO in stight excess. In optical properties it differs considerably from rhodon- ite. Out of ten specimens of bustamite and rhodonite, mostly from Franklin Furnace, that have been examined optically, two had properties very near those of the analyzed bustamite, six had properties near those of fowlerite, a variety of rhodonite, while one had properties about midway between the rhodonite and bustamite. ft, therefore, seemsprobable that bustamite is a subspecies of rhodonite with the approximate composition CaO.MnO.2SiOz. Further work to finally determine this is desir- able, and the authors would like to obtain samples of calciferous rhodonite and bustamite for optical study to determine to what extent replacement of MnO by CaO takes place in rhodonite. ALTERATION OF SILICATES BY SONSTADT'S SOLUTION T. -

GLY 4310C Lab 2 CLASS: SILICATE Subclass: Inosilicates Amphibole
GLY 4310C Lab 2 CLASS: SILICATE Subclass: Inosilicates Amphibole group Mineral Name H G Color Cleavage, Luster Other Notes Streak Color Fracture, or Properties Parting ANTHOPHYLLITE 5.5 3.3 purplish, uneven fracture vitreous to divergent habit brownish-gold/white silky TREMOLITE 4.5 2.4 white-beige/white splintery fracture vitreous LW-bright orange reticulated habit SW-dull orange TREMOLITE, variety Hexagonite 5.5 3.5 lavender, good basal cleavage silky reticulated habit dark green, yellow/white ACTINOLITE 5.5 3.5 gray green/ uneven fracture vitreous white HORNBLENDE 5.0 3.0 black/green 2 dirs. at 60 vitreous acid reaction due to calcite impurities GLAUCOPHANE 5.0 2.8 blue gray/blue uneven fracture silky to dull acid reaction due to calcite impurities RIEBECKITE 5.5 (3.5) black/black poor basal cleavage vitreous fibrous 1 GLY 4310C Lab 2 CLASS: SILICATE Subclass: Phyllosilicates Serpentine Group Mineral Name H G Color Cleavage, Luster Other Notes Streak Color Fracture, or Properties Parting ANTIGORITE 4.5 2.4 jade green/gray uneven fracture greasy slight acid associated with reaction due pyrite to impurity CHRYSOTILE (2.5) (3.2) white/white uneven fracture silky asbestiform GARNIERITE 5.0 purple/ jagged fracture vitreous light green Subclass: Phyllosilicates Clay Mineral Group Mineral Name H G Color Cleavage, Luster Other Notes Streak Color Fracture, or Properties Parting KAOLINITE 2.0 2.6 white/white uneven fracture earthy to sticks to tongue; does not greasy absorb liquid PYROPHYLLITE 2.5 2.9 silver,gold,red, splintery fracture vitreous -

Diamond Dan's Mineral Names Dictionary
A Dictionary of Mineral Names By Darryl Powell Mineral Names What do they mean? Who created them? What can I learn from them? This mineral diction‐ ary is unique because it is illustrated, both with mineral drawings as well as pictures of people and places after which some minerals are named. The people pictured on this page have all made a con‐ tribution to what is formally called “mineral nomenclature.” Keep reading and you will discover who they are and what they did. In 1995, Diamond Dan Publications pub‐ lished its first full book, “A Mineral Collector’s Guide to Common Mineral Names: Their Ori‐ gins & Meanings.” Now it is twenty years later. What you will discover in this issue and in the March issue is a re‐ vised and improved version of this book. This Mineral Names Dictionary contains mineral names that the average mineral collector will encounter while collecting minerals, attending shows and visiting museum displays. In addition to the most common min‐ eral names, there are some unofficial names which you will still find on labels. Each mineral name has a story to tell or a lesson to teach. If you wanted to take the time, each name could become a topic to study. Armalcolite, for example, could quickly be‐ come a study of a mineral, first discovered on the moon, and brought back to earth by the astronauts Armstrong, Aldrin and Collins (do you see parts of their names in this mineral name?) This could lead you to a study of American astronauts landing on the moon, what it took to get there and what we discovered by landing on the moon.