Features and Functioning of the Distal Forelimb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Articulationes Membri Thoracici • 1. Articulatio
ARTICULATIONES MEMBRI THORACICI • 1. ARTICULATIO HUMERI-art. simplex, art. spheroidea (but functions as a hinge joint) movement: eq, Ru only flexion, extension is possible, in ca: rotation, abduction, adduction also between scapula (cavitas glenoidalis) and humerus (caput) Capsula articularis Recessus: cranial and caudal recesses Labrum glenoidale Ligg. glenohumeralia (eq, ca)- tickened part of the capsule (capsular ligament) in the med. and lat. walls in ca, and cranially in eq Lig. coracohumerale (eq, Ru)- capsular ligament between scapula (tub. supraglenoidale) and humerus (tub. majus, minus) No collateral ligaments! Instead of them: laterally m. infraspinatus (1), medially m. subscapularis (5) ca: part of the joint capsule surrounds the tendon of m. biceps brachii (9) and forms vagina synovialis intertubercularis eq, bo: bursa intertubercularis (=bursa bicipitalis) under the tendor of the m. biceps brachii (may communicate with the joint cavity of the shoulder joint in horse) • 2.ARTICULATIO CUBITI-art. composita, ginglymus (hinge joint) movement: extension and flexion between humerus (condyle), radius (caput), ulna (insisura trochlearis) Articulatio humeroulnaris Articulatio humeroradialis Capsula articularis Recessus: recessus cranialis, large recessus caudalis Lig. collaterale cubiti mediale- from epicondylus med. to radius (in ca also to ulna) Lig. collaterale cubiti laterale- from epicondylus lat. to radius (in ca, Ru also to ulna) Lig. olecrani (ca)- capsular ligament from fossa olecrani of humerus to olecranon •3. ARTICULATIO RADIOULNARIS PROXIMALIS- art. simplex, art. trochoidea movement: ca: rotational movements are possible (pronatio, supinatio) eq, Ru: no movement! between radius (circumferentia articularis radii) and ulna (incisura radialis ulnae) Lig. anulare radii (ca)- encircles the head of the radius, running under the collateral ligaments Membrana interossea antebrachii (ca) (in eq, Ru it is ossified) • 4. -
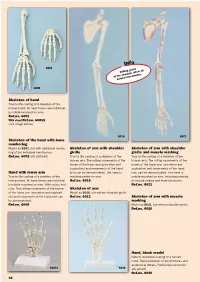
Skeleton of Hand Skeleton of the Hand with Bone Numbering Skeleton Of
Info 6001 Sliding joints at the shoulder allow all natural movements. 6008 Skeleton of hand True to life casting of a skeleton of the human hand. All hand bones are individual- ly mobile-mounted on wire. Ref.no. 6001 With stand Ref.no. 6001S (see image below) 6016 6021 Skeleton of the hand with bone numbering Model as 6001, but with additional numbe- Skeleton of arm with shoulder Skeleton of arm with shoulder ring of the individual hand bones. girdle girdle and muscle marking Ref.no. 6002 (not pictured) True to life casting of a skeleton of the True to life casting of a skeleton of the human arm. The rolling movements of the human arm. The rolling movements of the bones of the lower arm (pronation and bones of the lower arm (pronation and supination) and movements of the hand supination) and movements of the hand Hand with lower arm joint can be demonstrated. The hand is joint can be demonstrated. The hand is True to life casting of a skeleton of the mobilemounted on wire. mobile-mounted on wire. Including marking human hand. All hand bones are individual- Ref.no. 6016 of muscle origins and insertion points. ly mobile-mounted on wire. With radius and Ref.no. 6021 ulna. The rolling movements of the bones Skeleton of arm of the lower arm (pronation and supinati- Model as 6016, but without shoulder girdle. on) and movements of the hand joint can Ref.no. 6012 Skeleton of arm with muscle be demonstrated. marking Ref.no. 6008 Model as 6021, but without shoulder girdle Ref.no. -

Fins, Limbs, and Tails: Outgrowths and Axial Patterning in Vertebrate Evolution Michael I
Review articles Fins, limbs, and tails: outgrowths and axial patterning in vertebrate evolution Michael I. Coates1* and Martin J. Cohn2 Summary Current phylogenies show that paired fins and limbs are unique to jawed verte- brates and their immediate ancestry. Such fins evolved first as a single pair extending from an anterior location, and later stabilized as two pairs at pectoral and pelvic levels. Fin number, identity, and position are therefore key issues in vertebrate developmental evolution. Localization of the AP levels at which develop- mental signals initiate outgrowth from the body wall may be determined by Hox gene expression patterns along the lateral plate mesoderm. This regionalization appears to be regulated independently of that in the paraxial mesoderm and axial skeleton. When combined with current hypotheses of Hox gene phylogenetic and functional diversity, these data suggest a new model of fin/limb developmental evolution. This coordinates body wall regions of outgrowth with primitive bound- aries established in the gut, as well as the fundamental nonequivalence of pectoral and pelvic structures. BioEssays 20:371–381, 1998. 1998 John Wiley & Sons, Inc. Introduction over and again to exemplify fundamental concepts in biological Vertebrate appendages include an amazing diversity of form, theory. The striking uniformity of teleost pectoral fin skeletons from the huge wing-like fins of manta rays or the stumpy limbs of illustrated Geoffroy Saint-Hilair’s discussion of ‘‘special analo- frogfishes, to ichthyosaur paddles, the extraordinary fingers of gies,’’1 while tetrapod limbs exemplified Owen’s2 related concept aye-ayes, and the fin-like wings of penguins. The functional of ‘‘homology’’; Darwin3 then employed precisely the same ex- diversity of these appendages is similarly vast and, in addition to ample as evidence of evolutionary descent from common ances- various modes of locomotion, fins and limbs are also used for try. -

The Age Order of Epiphyseal Union Around Elbow Joint - a Radiological Study in Vidarbha
International Journal of Recent Trends in Science And Technology, ISSN 2277-2812 E-ISSN 2249-8109, Volume 10, Issue 2, 2014 pp 251-255 The Age Order of Epiphyseal Union around Elbow Joint - A Radiological Study in Vidarbha Nemade K. S.1*, Kamdi N. Y.2, Meshram M. M.3 1Assistant Professor, 2Associate Professor, 3Professor, Department of Anatomy, GMC, Nagpur, Maharashtra, INDIA. *Corresponding Address: [email protected] Research Article Abstract: Age of union of epiphysis is an important objective even by the workers from the various provinces of the method of age determination which is a difficult task for medico- Indian subcontinent ( Lal and Nat 1934 11 ; Pillai 1936 15 ; legal person. However, this age varies with racial, geographic, Galstaun 1937 9; Basu and Basu 1938 3,4 ; Lal and climatic and various other factors. Study of various text books in 12 et al 8 Anatomy and Radiology exhibits a glaring discrepancy as regards Townsend 1939 ; Gupta . 1974 ). Because of the the ages at which the different epiphyses fuse with the respective existence of such racial, geographic and climatic diaphyses in long bones. These variations have suggested need of variations, need for separate standards of ossification for separate standard of ossification for separate regions. This leads us separate regions have been suggested (Loder et al 1993 14 ; to study ages of epiphyseal union around elbow joint, a rarely Koc et al. 2001 10 ; Crowder et al. 2005 6). So, the present studied joint. Study was performed in total 320 healthy subjects work is undertaken as a pilot study to investigate the ages having ages from 13 to 23 years and length of residence in Vidarbh more than 10 years. -

Sesamoid Bone in the Tendon of the Supinator Muscle of Dogs: Incidence and Comparison of Radiographic and Computed Tomographic Features
Sesamoid bone in the tendon of the supinator muscle of dogs: incidence and comparison of radiographic and computed tomographic features Word count: 8473 Manon Dorny Student number: 01609678 Supervisor: Dr. Ingrid Gielen Supervisor: Prof. dr. Wim Van Den Broeck Supervisor: Dr. Aquilino Villamonte Chevalier A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Veterinary Medicine Academic year: 2018 - 2019 Ghent University, its employees and/or students, give no warranty that the information provided in this thesis is accurate or exhaustive, nor that the content of this thesis will not constitute or result in any infringement of third-party rights. Ghent University, its employees and/or students do not accept any liability or responsibility for any use which may be made of the content or information given in the thesis, nor for any reliance which may be placed on any advice or information provided in this thesis. ACKNOWLEDGEMENTS I would like to thank the people that helped me accomplish this thesis and helped me achieve my degree in veterinary science. First of all I would like to thank Dr. Ingrid Gielen, Dr. Aquilino Villamonte Chevalier and Prof. Dr. Wim Van Den Broeck. I thank them all for their time spend in helping me with my research, their useful advice and their endless patience. Without their help, I wouldn’t have been able to accomplish this thesis. Next I would like to thank my family and friends for their continuing support and motivation during the last years of vet school. My parents and partner especially, for all the mental breakdowns they had to endure in periods of exams and deadlines. -

Morphology and Evolution of Sesamoid Elements in Bats (Mammalia: Chiroptera)
Morphology and Evolution of Sesamoid Elements in Bats (Mammalia: Chiroptera) Author(s): http://orcid.org/0000-0002-7292-3256Lucila Inés Amador, Norberto Pedro Giannini, http://orcid.org/0000-0001-8807-7499Nancy B. Simmons and http:// orcid.org/0000-0002-4615-5011Virginia Abdala Source: American Museum Novitates, (3905):1-40. Published By: American Museum of Natural History https://doi.org/10.1206/3905.1 URL: http://www.bioone.org/doi/full/10.1206/3905.1 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. AMERICAN MUSEUM NOVITATES Number 3905, 38 pp. August 17, 2018 Morphology and Evolution of Sesamoid Elements in Bats (Mammalia: Chiroptera) LUCILA INÉS AMADOR,1 NORBERTO PEDRO GIANNINI,1, 2, 3 NANCY B. SIMMONS,2 AND VIRGINIA ABDALA4 ABSTRACT Sesamoids are skeletal elements found within a tendon or ligament as it passes around a joint or bony prominence. -

The Histology of Epiphyseal Union in Mammals
J. Anat. (1975), 120, 1, pp. 1-25 With 49 figures Printed in Great Britain The histology of epiphyseal union in mammals R. WHEELER HAINES* Visiting Professor, Department of Anatomy, Royal Free Hospital School of Medicine, London (Accepted 11 November 1974) INTRODUCTION Epiphyseal union may be defined as beginning with the completion of the first mineralized bridge between epiphyseal and diaphyseal bone and ending with the complete disappearance of the cartilaginous epiphyseal plate and its replacement by bone and marrow. The phases have been described by Sidhom & Derry (1931) and many others from radiographs, but histological material showing union in progress is rare, probably because of the rapidity with which union, once begun, comes to completion (Stephenson, 1924; Dawson, 1929). Dawson (1925, 1929) described the histology of 'lapsed union' in rats, where the larger epiphyses at the 'growing ends' of the long bones remain un-united through- out life. He and Becks et al. (1948) also discussed the early and complete type of union found at the distal end of the humerus in the rat. Here a single narrow per- foration pierced the cartilaginous plate near the olecranon fossa and later spread to destroy the whole plate. Lassila (1928) described a different type of union in the metatarsus of the calf, with multiple perforations of the plate. Apart from a few notes on human material (Haines & Mohiuddin, 1960, 1968), nothing else seems to have been published on the histology of union in mammals. In this paper more abundant material from dog and man is presented and will serve as a basis for discussion of the main features of the different types of union. -

FORELIMB LAMENESS: the GREAT IMPERSONATOR Juliette Hart, DVM, MS, CCRT, CVA Cornell University Veterinary Specialists
FORELIMB LAMENESS: THE GREAT IMPERSONATOR Juliette Hart, DVM, MS, CCRT, CVA Cornell University Veterinary Specialists. Stamford, CT Diagnosis of forelimb lameness in canine patients can often be a labor-intensive and time- consuming process, often with multiple factors being taken into account, regardless of the actual diagnosis. The dog’s age, activity level, co-morbidities, job and environment can be key players. Close examination of the dog in motion (in hospital and at home) can be helpful when determining type and degree of lameness, and may frequently assist the clinician in determining next appropriate diagnostic tests and treatment plans. This lecture will focus on differentials associated with forelimb lameness in dogs, current diagnostic tests and potential treatments available, and finally prognoses and outcomes for specific types of shoulder forelimb lameness in dogs. Lameness Evaluation The forelimb skeleton consists of the thoracic or pectoral girdle and the bones of the forelimb. The canine scapula itself is positioned close to the sagittal plane, and the humeral head is less rounded (as compared to the human head) to assist with weight bearing. The radius takes the majority of weight-bearing in the antebrachium. And, although small, the many sesamoid bones in the carpus/paw allow for biomechanically advantageous alignment of angles of insertion of tendons at their attachments.¹ While there can be tremendous variation in the sizes of the bones themselves comparing dog to dog, the literature have reported a roughly 60% body weight distribution in the thoracic limbs.² As a clinician evaluates a patient, lameness is a key element of that examination. -

From Fin to Forelimb Crucially Showing That They Develop in Situ Rather Than Migrating to Their the Vertebrate Invasion of Land Was Cartilaginous Fish Such As Sharks
NATURE|Vol 466|5 August 2010 NEWS & VIEWS Goulielmakis and colleagues1 characterized Figure 1 | The first attosecond probe the coherence, and thus the entanglement, of experiments. Goulielmakis et al.1 report a Kr+ and the lost electron. In their experiments, technique for observing electron motion in the intense, ultrashort pump pulse ensures real time. They irradiated krypton atoms (Kr) significant overlap of the two quantum states Kr+, 3d–1 with a ‘pump’ pulse of infrared light lasting a few femtoseconds, liberating electrons to of the removed electron that correlate with generate Kr+ ions in a superposition of two two different pathways in the ion’s subsystem states, 4p−1(J = 1/2) and 4p−1(J = 3/2), where J is (Fig. 1b), resulting in a low electron–ion entan- total angular momentum. Black arrows indicate glement, a high coherence of the hole’s wave the two ionization pathways. The authors then packet and high visibility of the interference Kr+, irradiated the ions with attosecond ‘probe’ pulses 4p–1(J=1/2) fringes. The ability to probe decoherence is a + of extreme-ultraviolet light, exciting them to a Kr , −1 very important aspect of the experiment. 4p–1(J=3/2) higher-energy 3d state; red and green arrows The authors’ experiment is reminiscent of a indicate the two possible excitation pathways. two-colour coherent-control scheme2. In such The complete system constitutes an entangled electron–ion pair. a, The different excitation schemes, population of a final state is controlled pathways taken by the ion to reach the 3d−1 by the relative phase between the two colours state may cause the liberated electrons to adopt of light needed to promote a system from two orthogonal quantum states. -

Sesamoid Bone of the Medial Collateral Ligament of the Knee Joint
CASE REPORT Eur. J. Anat. 21 (4): 309-313 (2017) Sesamoid bone of the medial collateral ligament of the knee joint Omar M. Albtoush, Konstantin Nikolaou, Mike Notohamiprodjo Department of Diagnostic and Interventional Radiology, Karls Eberhard Universität Tübingen, Hoppe-Seyler-Str. 3, 72076 Tübingen, Germany SUMMARY tomical relations and the exclusion of other possi- bilities. The variable occurrence of the sesamoid bones This article supports the theory stating that the supports the theory stating that the development development and evolution of the sesamoid bones and evolution of these bones are controlled are controlled through the interaction between in- through the interaction between intrinsic genetic trinsic genetic factors and extrinsic epigenetic stim- factors and extrinsic stimuli. In the present article uli, which can explain their variable occurrence. we report a sesamoid bone at the medial collateral ligament of the knee joint, a newly discovered find- CASE REPORT ing in human and veterinary medicine. We present a case of a 51-year-old female pa- Key words: Sesamoid – MCL – Knee – Fabella – tient, who presented with mild pain at the medial Cyamella aspect of the left knee. No trauma has been re- ported. An unenhanced spiral CT-Scan was per- INTRODUCTION formed with 2 mm thickness, 120 kvp and 100 mAs, which showed preserved articulation of the New structural anatomical discoveries are not so knee joint with neither joint effusion, nor narrowing often encountered. However, their potential occur- of the joint space nor articulating cortical irregulari- rence should be kept in mind, which can eventually ties (Fig. 1). Mild subchondral sclerosis was de- help in a better understanding of patients’ symp- picted at the medial tibial plateau as a sign of early toms and subsequently improve the management osteoarthritis. -

Tetrapod Limb and Sarcopterygian Fin Regeneration Share a Core Genetic
ARTICLE Received 28 Apr 2016 | Accepted 27 Sep 2016 | Published 2 Nov 2016 DOI: 10.1038/ncomms13364 OPEN Tetrapod limb and sarcopterygian fin regeneration share a core genetic programme Acacio F. Nogueira1,*, Carinne M. Costa1,*, Jamily Lorena1, Rodrigo N. Moreira1, Gabriela N. Frota-Lima1, Carolina Furtado2, Mark Robinson3, Chris T. Amemiya3,4, Sylvain Darnet1 & Igor Schneider1 Salamanders are the only living tetrapods capable of fully regenerating limbs. The discovery of salamander lineage-specific genes (LSGs) expressed during limb regeneration suggests that this capacity is a salamander novelty. Conversely, recent paleontological evidence supports a deeper evolutionary origin, before the occurrence of salamanders in the fossil record. Here we show that lungfishes, the sister group of tetrapods, regenerate their fins through morphological steps equivalent to those seen in salamanders. Lungfish de novo transcriptome assembly and differential gene expression analysis reveal notable parallels between lungfish and salamander appendage regeneration, including strong downregulation of muscle proteins and upregulation of oncogenes, developmental genes and lungfish LSGs. MARCKS-like protein (MLP), recently discovered as a regeneration-initiating molecule in salamander, is likewise upregulated during early stages of lungfish fin regeneration. Taken together, our results lend strong support for the hypothesis that tetrapods inherited a bona fide limb regeneration programme concomitant with the fin-to-limb transition. 1 Instituto de Cieˆncias Biolo´gicas, Universidade Federal do Para´, Rua Augusto Correa, 01, Bele´m66075-110,Brazil.2 Unidade Genoˆmica, Programa de Gene´tica, Instituto Nacional do Caˆncer, Rio de Janeiro 20230-240, Brazil. 3 Benaroya Research Institute at Virginia Mason, 1201 Ninth Avenue, Seattle, Washington 98101, USA. 4 Department of Biology, University of Washington 106 Kincaid, Seattle, Washington 98195, USA. -

Evolution of the Muscular System in Tetrapod Limbs Tatsuya Hirasawa1* and Shigeru Kuratani1,2
Hirasawa and Kuratani Zoological Letters (2018) 4:27 https://doi.org/10.1186/s40851-018-0110-2 REVIEW Open Access Evolution of the muscular system in tetrapod limbs Tatsuya Hirasawa1* and Shigeru Kuratani1,2 Abstract While skeletal evolution has been extensively studied, the evolution of limb muscles and brachial plexus has received less attention. In this review, we focus on the tempo and mode of evolution of forelimb muscles in the vertebrate history, and on the developmental mechanisms that have affected the evolution of their morphology. Tetrapod limb muscles develop from diffuse migrating cells derived from dermomyotomes, and the limb-innervating nerves lose their segmental patterns to form the brachial plexus distally. Despite such seemingly disorganized developmental processes, limb muscle homology has been highly conserved in tetrapod evolution, with the apparent exception of the mammalian diaphragm. The limb mesenchyme of lateral plate mesoderm likely plays a pivotal role in the subdivision of the myogenic cell population into individual muscles through the formation of interstitial muscle connective tissues. Interactions with tendons and motoneuron axons are involved in the early and late phases of limb muscle morphogenesis, respectively. The mechanism underlying the recurrent generation of limb muscle homology likely resides in these developmental processes, which should be studied from an evolutionary perspective in the future. Keywords: Development, Evolution, Homology, Fossils, Regeneration, Tetrapods Background other morphological characters that may change during The fossil record reveals that the evolutionary rate of growth. Skeletal muscles thus exhibit clear advantages vertebrate morphology has been variable, and morpho- for the integration of paleontology and evolutionary logical deviations and alterations have taken place unevenly developmental biology.