Microwave-Assisted Synthesis of Amikacin Modified N,S Co-Doped
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OSB Representative Participant List by Industry
OSB Representative Participant List by Industry Aerospace • KAWASAKI • VOLVO • CATERPILLAR • ADVANCED COATING • KEDDEG COMPANY • XI'AN AIRCRAFT INDUSTRY • CHINA FAW GROUP TECHNOLOGIES GROUP • KOREAN AIRLINES • CHINA INTERNATIONAL Agriculture • AIRBUS MARINE CONTAINERS • L3 COMMUNICATIONS • AIRCELLE • AGRICOLA FORNACE • CHRYSLER • LOCKHEED MARTIN • ALLIANT TECHSYSTEMS • CARGILL • COMMERCIAL VEHICLE • M7 AEROSPACE GROUP • AVICHINA • E. RITTER & COMPANY • • MESSIER-BUGATTI- CONTINENTAL AIRLINES • BAE SYSTEMS • EXOPLAST DOWTY • CONTINENTAL • BE AEROSPACE • MITSUBISHI HEAVY • JOHN DEERE AUTOMOTIVE INDUSTRIES • • BELL HELICOPTER • MAUI PINEAPPLE CONTINENTAL • NASA COMPANY AUTOMOTIVE SYSTEMS • BOMBARDIER • • NGC INTEGRATED • USDA COOPER-STANDARD • CAE SYSTEMS AUTOMOTIVE Automotive • • CORNING • CESSNA AIRCRAFT NORTHROP GRUMMAN • AGCO • COMPANY • PRECISION CASTPARTS COSMA INDUSTRIAL DO • COBHAM CORP. • ALLIED SPECIALTY BRASIL • VEHICLES • CRP INDUSTRIES • COMAC RAYTHEON • AMSTED INDUSTRIES • • CUMMINS • DANAHER RAYTHEON E-SYSTEMS • ANHUI JIANGHUAI • • DAF TRUCKS • DASSAULT AVIATION RAYTHEON MISSLE AUTOMOBILE SYSTEMS COMPANY • • ARVINMERITOR DAIHATSU MOTOR • EATON • RAYTHEON NCS • • ASHOK LEYLAND DAIMLER • EMBRAER • RAYTHEON RMS • • ATC LOGISTICS & DALPHI METAL ESPANA • EUROPEAN AERONAUTIC • ROLLS-ROYCE DEFENCE AND SPACE ELECTRONICS • DANA HOLDING COMPANY • ROTORCRAFT • AUDI CORPORATION • FINMECCANICA ENTERPRISES • • AUTOZONE DANA INDÚSTRIAS • SAAB • FLIR SYSTEMS • • BAE SYSTEMS DELPHI • SMITH'S DETECTION • FUJI • • BECK/ARNLEY DENSO CORPORATION -

Retirement Strategy Fund 2060 Description Plan 3S DCP & JRA
Retirement Strategy Fund 2060 June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA ACTIVIA PROPERTIES INC REIT 0.0137% 0.0137% AEON REIT INVESTMENT CORP REIT 0.0195% 0.0195% ALEXANDER + BALDWIN INC REIT 0.0118% 0.0118% ALEXANDRIA REAL ESTATE EQUIT REIT USD.01 0.0585% 0.0585% ALLIANCEBERNSTEIN GOVT STIF SSC FUND 64BA AGIS 587 0.0329% 0.0329% ALLIED PROPERTIES REAL ESTAT REIT 0.0219% 0.0219% AMERICAN CAMPUS COMMUNITIES REIT USD.01 0.0277% 0.0277% AMERICAN HOMES 4 RENT A REIT USD.01 0.0396% 0.0396% AMERICOLD REALTY TRUST REIT USD.01 0.0427% 0.0427% ARMADA HOFFLER PROPERTIES IN REIT USD.01 0.0124% 0.0124% AROUNDTOWN SA COMMON STOCK EUR.01 0.0248% 0.0248% ASSURA PLC REIT GBP.1 0.0319% 0.0319% AUSTRALIAN DOLLAR 0.0061% 0.0061% AZRIELI GROUP LTD COMMON STOCK ILS.1 0.0101% 0.0101% BLUEROCK RESIDENTIAL GROWTH REIT USD.01 0.0102% 0.0102% BOSTON PROPERTIES INC REIT USD.01 0.0580% 0.0580% BRAZILIAN REAL 0.0000% 0.0000% BRIXMOR PROPERTY GROUP INC REIT USD.01 0.0418% 0.0418% CA IMMOBILIEN ANLAGEN AG COMMON STOCK 0.0191% 0.0191% CAMDEN PROPERTY TRUST REIT USD.01 0.0394% 0.0394% CANADIAN DOLLAR 0.0005% 0.0005% CAPITALAND COMMERCIAL TRUST REIT 0.0228% 0.0228% CIFI HOLDINGS GROUP CO LTD COMMON STOCK HKD.1 0.0105% 0.0105% CITY DEVELOPMENTS LTD COMMON STOCK 0.0129% 0.0129% CK ASSET HOLDINGS LTD COMMON STOCK HKD1.0 0.0378% 0.0378% COMFORIA RESIDENTIAL REIT IN REIT 0.0328% 0.0328% COUSINS PROPERTIES INC REIT USD1.0 0.0403% 0.0403% CUBESMART REIT USD.01 0.0359% 0.0359% DAIWA OFFICE INVESTMENT -

Mass Spectrometer Business Presentation Materials
Mass Spectrometer Business Presentation Materials Hiroto Itoi, Corporate Officer Deputy General Manager of the Analytical & Measuring Instruments Division Shimadzu Corporation Jul. 3, 2018 Contents I. Introduction • Expansion of Mass Spectrometry ………………………………………………………………… p.3 • History of Shimadzu's Growth in Mass Spectrometry …………………………………………… p.5 II. Overview of Mass Spectrometers • Operating Principle, Demand Trends, and Vendors ……………………………………………… p.9 • Mass Spectra ………………………………………………………………………………………… p.10 • Configuration of Mass Spectrometers …………………………………………………………… p.11 • Ionization …………………………………………………………………………………………… p.12 • Mass Separation …………………………………………………………………………………… p.14 III. Shimadzu's Mass Spectrometer Business • Product Type ………………………………………………………………………………………… p.17 • Application Software ………………………………………………………………………………… p.18 • Growth Strategy for Mass Spectrometer Business ……………………………………………… p.19 • Expand/Improve Product Lines …………………………………………………………………… p.20 • Measures to Expand Application Fields …………………………………………………………… p.24 • Measures to Automate Data Processing Using AI ……………………………………………… p.25 IV. Summary • Future Direction ……………………………………………………………………………………… p.26 July 2018 Mass Spectrometer Business Presentation Materials 2 I. Introduction Expansion of Mass Spectrometry (1) Why Mass Spectrometry? Mass spectrometry is able to analyze a wide variety of compounds with high accuracy and high efficiency (simultaneous multicomponent analysis). It offers superior characteristics that are especially beneficial in the following fields, -

Volume 39 Number 2, 2004
Volume 39 number 2, 2004 ISSN 0385-4426 water Volume 39 Number 2 TEM Study of Water in Carbon November 30, 2004 Nanotubes Haihui Ye 1, Nevin Naguib, and Yury Gogotsi 2 Department of Materials Science and Engineering and A. J. Drexel Nanotechnology Institute, Drexel University High resolution transmission electron microscopy (HRTEM) and electron ener- gy loss spectroscopy (EELS), supported by TEM simulation, show the presence of water in carbon nanotubes (CNT) subjected to hydrothermal treatment. It was observed that water behaves differently in the channels of CNT with different inner diameters. In large CNT (50 - 200 nm in diameter) water behaves fairly con- ventionally, showing a well-defined meniscus. Rapid evaporation and condensa- tion upon the intensity change of electron beam, as well as strong interaction between nanotube walls and water, have been observed. Water in small CNT with inner diameter of 2 - 5 nm shows a surprisingly high contrast in HRTEM images. Fluidity of water in such small channels is greatly retarded compared to Contents the macroscale. Both beam heating and electron bombardment in TEM play important role and affect water behavior in the small CNT. The present findings TEM Study of Water pose new challenges for modeling and device development. in Carbon Nanotubes . 2 A Study of Metal Nanowire Structures by High-Resolution Transmission Electron Microscopy. 8 Introduction dered, forming a hydrophobic or hydrophilic Introduction inner surface respectively, which makes CNT of JWS-2000 Review SEM . 13 The properties of water in confinement dif- an excellent container to study the interaction Grazing-Exit Electron Probe fer from those of bulk water [1-3]. -

Registered X-Ray Vendors Department of Radiological Health
Department of Radiological Health Type: All Registered X-ray Vendors Address Type:Business Class: CLASS II Class: CLASS II Installation or Servicing of Radiation Machines and Associated Companies Type: Bone Density Reg. # Name Address Contact Person Phone 00-0264 BLUE RIDGE X-RAY GREER 1361 W WADE HAMPTON BLVD STE F #109 GREER, SC 29650 PAUL EILL 800-727-7290 00-0044 GE HEALTHCARE 9900 INNOVATION DR MAILCODE RP2176 WAUWATOSA, WI 53226 BILL GIBSON 00-0184 HOLOGIC INC 36 APPLE RIDGE RD DANBURY, CT 06810 LIDA REED 781-999-7589 00-5183 INTEGRITY MEDICAL SYSTEMS INC 13831 JETPORT COMMERCE PKWY STE A FORT MYERS, FL 33913 00-5226 POOLE & ASSOCIATES 214 BEREA HEIGHTS RD GREENVILLE, SC 29610 JOSEPH POOLE 864-320-7286 Type: CT Reg. # Name Address Contact Person Phone 00-0044 GE HEALTHCARE 9900 INNOVATION DR MAILCODE RP2176 WAUWATOSA, WI 53226 BILL GIBSON Type: Dental Reg. # Name Address Contact Person Phone 00-5218 ASSOCIATED DENTAL SPECIALISTS INC 95 LOBLOLLY LN BLAIRSVILLE, GA 30512 00-0055 ATLANTA DENTAL SUPPLY 1650 SATELLITE BLVD DULUTH, GA 30097 KEN BEST 770-455-4444 00-5007 BENCO DENTAL CO 295 CENTERPOINT BLVD PITTSTON, PA 18640 TERRY MOORE 770-640-1150 00-5007 BENCO DENTAL CO 295 CENTERPOINT BLVD PITTSTON, PA 18640 CURLEY NEWTON 828-428-9438 00-5007 BENCO DENTAL CO 295 CENTERPOINT BLVD PITTSTON, PA 18640 CLEM SUDER 770-640-1150 00-5471 CAPITAL OF TEXAS DENTAL SOLUTIONS LLC 2001 WINDY TERRACE STE F CEDAR PARK, TX 78613 JAKE LEBENS 512-270-5199 00-5180 DENTAL IMAGING TECHNOLOGIES CORPORATION 1910 N PENN RD HATFIELD, PA 19440 DAVE COWAN 267-483-8087 -
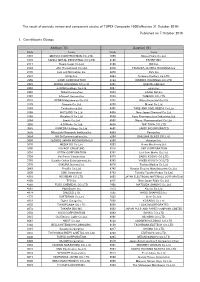
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

Service Listing Class I and II Sales and Service
Service Listing Class I and II Sales and Service 999-S00108420/20 IMAGING A SUBDIVISION OF KMHA JAN MANISCALCO 829 S VIRGINIA RD STE A CRYSTAL LAKE, IL 60014-8714 8009341034 [email protected] CLASS_I Medical (Radiographic) CLASS_II Medical (Radiographic) 999-S001033A G MEDICAL EQUIPMENT INC ALAN D RUSK 147 CHESTATEE SPRINGS RIDGE DAHLONEGA, GA 30533-6146 6784100573 [email protected] CLASS_I Medical (Fluoroscopic) Medical (Radiographic) Veterinary CLASS_II Medical (Fluoroscopic) Medical (Radiographic) Veterinary 999-S000862A+ MEDICAL COMPANY INC JEANETTE BOTKO 1679 OVERVIEW DR STE 101 ROCK HILL, SC 29732-7469 8032339148 [email protected] CLASS_I Sales Other CLASS_II Medical (Fluoroscopic) Medical (Radiographic) 999-S000156ABB INC RADIOLOGICAL OPERATIONS JONATHAN C FORTKAMP PH D RSO 579 EXECUTIVE CAMPUS DR WESTERVILLE, OH 43082-8870 6148186300 [email protected] CLASS_I Wednesday, April 1, 2020 Page 1 of 73 Non-Healing Arts (Industrial Radiography) CLASS_II Non-Healing Arts (Industrial Radiography) 41-S001137 ABSOLUTE IMAGING SOLUTIONS ANDREA LOVELL DIRECTOR OF OPERATIONS PO BOX 687 OAK RIDGE, NC 27310-0687 3366432000 [email protected] CLASS_I Medical (Radiographic) CLASS_II Medical (Radiographic) 999-S000887ACCELETRONICS SERVICE INC COLEEN BARBEZAT 602 GORDON DR EXTON, PA 19341-1261 6105243300 [email protected] CLASS_I Medical (Radiographic) Medical (Therapeutic) Non-Healing Arts (Accelerators) Non-Healing Arts (Industrial Radiography) Veterinary CLASS_II Medical (Radiographic) Medical (Therapeutic) -

Istoxx® Mutb Japan Momentum 300 Index
ISTOXX® MUTB JAPAN MOMENTUM 300 INDEX Components1 Company Supersector Country Weight (%) Z HOLDINGS Technology Japan 0.69 M3 Health Care Japan 0.65 KOEI TECMO HOLDINGS Technology Japan 0.65 MENICON Health Care Japan 0.59 CAPCOM Technology Japan 0.58 FUJITEC Industrial Goods & Services Japan 0.56 Ibiden Co. Ltd. Industrial Goods & Services Japan 0.56 NIPPON PAINT HOLDINGS Chemicals Japan 0.56 RENESAS ELECTRONICS Technology Japan 0.55 JEOL Industrial Goods & Services Japan 0.55 INTERNET INTV.JAPAN Technology Japan 0.53 JSR Corp. Chemicals Japan 0.52 NET ONE SYSTEMS Technology Japan 0.51 Fujitsu Ltd. Technology Japan 0.51 Bank of Kyoto Ltd. Banks Japan 0.51 Hokuhoku Financial Group Inc. Banks Japan 0.51 FUJITSU GENERAL Personal & Household Goods Japan 0.50 Iyo Bank Ltd. Banks Japan 0.50 Kyushu Financial Group Banks Japan 0.50 77 Bank Ltd. Banks Japan 0.49 COCOKARA FINE INC. Retail Japan 0.49 TOSHIBA TEC Industrial Goods & Services Japan 0.48 JCR PHARMACEUTICALS Health Care Japan 0.48 MONOTARO Retail Japan 0.48 COSMOS PHARM. Retail Japan 0.48 Tokyo Electron Ltd. Technology Japan 0.48 Nomura Research Institute Ltd. Technology Japan 0.48 Olympus Corp. Health Care Japan 0.47 SUNDRUG Retail Japan 0.47 Chiba Bank Ltd. Banks Japan 0.47 NEC NETWORKS & SY.INTG. Technology Japan 0.47 Nomura Holdings Inc. Financial Services Japan 0.47 TOKYO OHKA KOGYO Technology Japan 0.47 PENTA-OCEAN CONSTRUCTION Construction & Materials Japan 0.47 FUYO GENERAL LEASE Financial Services Japan 0.46 FUJI Industrial Goods & Services Japan 0.46 Hachijuni Bank Ltd. -

JASIS 2019 Exhibitors
JASIS 2019 Exhibitors Elemental Scientic, Inc. A Elementar Japan K.K. a priori Inc. ELGA LabWater A&D Company, Limited ELIONIX INC. ACTAC.CO.,LTD Emerging Technologies Corporation. Acumentech Co., Ltd. entex inc. AD Science Inc. Eppendorf Co., Ltd. Advanced Energy Japan K.K. ERECTA International Corporation Advantec Toyo Kaisha, Ltd. ESPEC CORP. ADVANTEST CORPORATION ESPEC MIC CORP. Agilent Technologies Japan,Ltd. ETRI AINEX CO., LTD. Excimer,inc AIRIX Corp. Airtech Corporation AIVS Corporation F Alpha M.O.S. Japan K.K. Filgen, Inc. AMETEK Co, Ltd FLON CHEMICAL INC. AMR, Inc. FLON INDUSTRY CO., LTD. analytica-Messe Muenchen ForDx, Inc. Analytik Jena Japan Co., Ltd. Forum for Innovative Regenerative Medicine(FIRM) ANRITSU METER CO., LTD. FUJIKIN Incorporated Anton Paar Japan K.K. Fujitsu Limited Aomori Prefecture Quantum Science Center FUKUSHIMA INDUSTRIES CORP. APRO Science Institute, Inc. FUSO Co., Ltd. ARAM CORPORATION FUTA-Q,Ltd. AS ONE CORPORATION ASAHI KOHSAN Co.,Ltd Asahi Lab Commerce, Inc. G ASAHI LIFE SCIENCE CO.,LTD GASTEC CORPORATION ASAHI RUBBER INC. GC INSTRUMENTS ASAHI TECHNEION CO., LTD. GE Healthcare Japan Corporation ASCH JAPAN CO.,LTD GERSTEL K.K. ASI-AURORA GL Sciences Inc. ASICON Tokyo Ltd. Glass Expansion Pty. Ltd. Association for structure characterization Global Facility Center,HOKKAIDO UNIVERSITY ASTECH CORPORATION GTR TEC CORPORATION ATAGO CO., LTD. GVS Japan K.K. B H BAS Inc. hagataya.co.ltd BD Consulting L.L.C. Hakuto Co., Ltd. BeatSensing co.,ltd. HAMAMATSU PHOTONICS K.K. Beckman Coulter K.K. Hamilton Company Japan K.K. BEIJING LINGGONG TECHNOLOGY CO.,LTD Hanaichi UltraStructure Research Institute BETHL Co.,Ltd. -

Nano・Picometer Measurement and Control Laboratory
Mechanical Engineering 1 Nano・picometer measurement and control laboratory Professor / Masato AKETAGAWA Assistant Professor / Wei DONG Technical Staff / Masahiro YOSHIDA http://mcweb.nagaokaut.ac.jp/~picotech/ Can not make things that can not be measured. We are conducting ultra precision measurement using light (laser). A laboratory that respects the autonomy of students, it is a very free laboratory. Professor / Masato AKETAGAWA, Assistant Professor / Wei DONG Supervisor Professor Aketagawa has the experience of being a researcher at a company. Therefore, he has wide and deep knowledge about optical measurement technology. We encourage students to study in abroad, respecting our independence, and leading research with precise advice.Assistant Professor Wei always provide us various topics not only also a campus life. Research Content Mechanical In our laboratory we are researching ultra precision measurement system using leaser. We aim to contribute to the development of nanotechnology by considering a method of precision measurement, precision control and precision processing in nano (1 / billionth ) pico (1 / trillion) meter Electrical order. [1] Frequency stabilization and displacement measurement of 780nm DBR laser diode. [2]Ultra precision measurement of refractive index of air. [3] Measurement of motion error of bearing: Precise measurement of all 5 degrees of freedom bearing error by all simultaneously. Appearance of improved linear scale Materials [4]Length measurement with optical comb. calibrator A Day in the Lab Civil In our laboratory,no core time, and students has a great responsbility for a management of there resaech. Some people come early in the morning and are studying, others are still late at night. The seminars that are held twice a week are seminars progress seminar and paper seminar. -

Tax Evasion and Weapon Production Mailbox Arms Companies in the Netherlands
Issue Brief – May 2016 Tax evasion and weapon production Mailbox arms companies in the Netherlands Martin Broek Stop Wapenhandel www.stopwapenhandel.org Tax evasion and weapon production | 1 AUTHOR: Martin Broek EDITORS: Nick Buxton and Wendela de Vries DESIGN: Evan Clayburg Published by Transnational Institute – www.TNI.org and Stop Wapenhandel – www.StopWapenhandel.org Contents of the report may be quoted or reproduced for non-commercial purposes, provided that the source of information is properly cited. TNI would appreciate receiving a copy or link of the text in which this document is used or cited. Please note that for some images the copyright may lie elsewhere and copyright conditions of those images should be based on the copyright terms of the original source. http://www.tni.org/copyright ACKNOWLEDGEMENTS This is an updated briefing, initially released in January 2016. Tax evasion and weapon production | 2 Contents Introduction 4 Chapter 1: Short history of Dutch tax law 6 Chapter 2: Tax evasion in the Netherlands 8 Chapter 3: Top 10 defence industries and Dutch holdings 11 Chapter 4: Tax evasion by company 14 Chapter 5: Corruption and misbehaviour 27 Chapter 6: The Dutch connection in the Malaysian airline disaster 29 Chapter 7: Panama Papers and the arms trade 32 Conclusion 35 Annex – The use of Trusts 36 Notes 37 Tax evasion and weapon production | 3 Introduction The revelations of the leaked Panama Papers in April 2016 pushed the issue of tax and tax evasion high up the international political agenda. Prompting scandals and high profile resignations, the 11.5 million documents from the offshore law firm Mossack Fonseca unveiled some of the tricks and strategies that countless politicians, businessmen and elites use to avoid taxes. -

Istoxx® Mutb Japan Momentum 300 Index
ISTOXX® MUTB JAPAN MOMENTUM 300 INDEX Components1 Company Supersector Country Weight (%) SHIMACHU Retail Japan 0.83 LASERTEC Technology Japan 0.66 KADOKAWA Media Japan 0.65 KOEI TECMO HOLDINGS Technology Japan 0.64 M3 Health Care Japan 0.64 Taiyo Yuden Co. Ltd. Technology Japan 0.64 BAYCURRENT CONSULTING Technology Japan 0.63 AZBIL CORP. Industrial Goods & Services Japan 0.59 TOKYO OHKA KOGYO Technology Japan 0.58 DAIWABO HOLDINGS Industrial Goods & Services Japan 0.58 Ibiden Co. Ltd. Technology Japan 0.57 JEOL Industrial Goods & Services Japan 0.56 IR JAPAN HOLDINGS Industrial Goods & Services Japan 0.56 FUJI Industrial Goods & Services Japan 0.55 Tokyo Electron Ltd. Technology Japan 0.55 NIHON M&A CENTER Financial Services Japan 0.55 SUSHIRO GLOBAL HDG. Travel & Leisure Japan 0.55 Tokyo Century Corp Financial Services Japan 0.55 Advantest Corp. Technology Japan 0.54 CAPCOM Consumer Products & Services Japan 0.54 DAIFUKU Industrial Goods & Services Japan 0.54 NEXON Consumer Products & Services Japan 0.53 NIPPON GAS Utilities Japan 0.53 MONOTARO Retail Japan 0.52 TDK Corp. Technology Japan 0.50 TOKYO SEIMITSU Technology Japan 0.50 SHIFT Technology Japan 0.49 NIPPON PAINT HOLDINGS Industrial Goods & Services Japan 0.49 Sumco Corp. Technology Japan 0.48 Toho Gas Co. Ltd. Utilities Japan 0.48 KOBE BUSSAN Personal Care, Drug & Grocery Japan 0.48 Murata Manufacturing Co. Ltd. Technology Japan 0.47 Nomura Research Institute Ltd. Technology Japan 0.47 GMO PAYMENT GTWY. Industrial Goods & Services Japan 0.47 MIURA Industrial Goods & Services Japan 0.47 Obic Co. Ltd.