Territorial and Mating Success of Dragonflies That Vary in Muscle Power Output and Presence of Gregarine Gut Parasites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dragonflies September 2008
Learning Laguna Field Notes: Dragonflies September 2008 This edition is based on a Continuing Education event taught by Kathy & Dave Biggs. Dragonfly is a type of insect belonging to the order Odonata. As such, they have 3 prominent body parts—a head, a thorax to which the wings (in this case 4) and 6 legs are attached, and an abdomen. Their front and rear wings are not linked together, but can be operated independently. It is characterized by large multifaceted eyes, two pairs of strong, transparent wings, and an elongated body. Dragonflies typi- cally eat gnats, mosquitoes, and other small insects like flies, bees, and even butterflies. They are valued as predators, since they help control populations of potentially harmful insects and “pests.” Eight Spotted Skimmer - male (Libellula forensis) Mark A. Chappell Some Dragonfly “Wow” Facts http://faculty.ucr.edu/~chappell/INW/arthropods/8spot1.jpg • Because of their compound eyes, they can see in all directions and can see flickers of movement 15 times better than humans. • Predators like birds, especially the Black Phoebe, will eat them, but will drop the wings as they have barbs • In most dragonfly species, the eyes touch • Their legs are studded with spikes and they capture prey by clasping it in their legs. • Dragonflies usually attack prey from below • Like most bird species, males are more colorful that females • In the United States dragonflies and damselflies are sought out as a hobby similar to birding and butterflying, known as oding. Oding, from the dragonfly’s scientific order name: Odo- nata. • Dragonflies are survivors from before the dinosaur period. -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Checklist Dragonfly and Damselfly
To report sightings, contact: Natural Resources Coordinator 980-314-1119 www.parkandrec.com DRAGONFLY AND DAMSELFLY CHECKLIST Mecklenburg County, NC: 88 species Petaltails ☐ Swift River Crusier ☐ Autumn Meadowhawk ☐ Gray Petaltail (Tachopteryx thoreyi)*∆ (Macromia illinoiensis)*∆ (Sympetrum vicinum)*∆ ☐ Royal River Cruiser ☐ Carolina Saddlebags (Tramea carolina)*∆ Darners (Macromia taeniolata)*∆ ☐ Black Saddlebags (Tramea lacerata)*∆ ☐ Shadow Darner (Aeshna umbrosa) ☐ Common Green Darner (Anax junius)*∆ Emeralds Broad-winged Damsels ☐ Comet Darner (Anax longipes)*∆ ☐ Common Baskettail (Epitheca cynosura)*∆ ☐ Sparkling Jewelwing ☐ Springtime Darner (Basiaeschna janata)* ☐ Prince Baskettail (Epitheca princeps)*∆ (Calopteryx dimidiata)* ☐ Fawn Darner (Boyeria vinosa) ☐ Selys’ Sundragon (Helocordulia selysii) ☐ Ebony Jewelwing (Calopteryx maculata)*∆ ☐ Swamp Darner (Epiaeschna heros)*∆ ☐ Mocha Emerald (Somatochlora linearis)*∆ ☐ Smoky Rubyspot (Hetaerina titia) ☐ Taper-tailed Darner ☐ Clamp-tipped Emerald Spreadwings (Gomphaeschna antilope)*∆ (Somatochlora tenebrosa)*∆ ☐ Elegant Spreadwing (Lestes inaequalis)* ☐ Cyrano Darner Skimmers ☐ Southern Spreadwing (Lestes australis) (Nasiaeschna pentacantha)*∆ ☐ Four-spotted Pennant ☐ Amber-winged Spreadwing Clubtails (Brachymesia gravida)*∆ (Lestes eurinus)* ☐ Two-striped Forceptail ☐ Calico Pennant (Celithemis elisa)*∆ ☐ Slender Spreadwing (Lestes rectangularis)* (Aphylla williamsoni)*∆ ☐ Halloween Pennant (Celithemis eponina)*∆ ☐ Swamp Spreadwing (Lestes vigilax) ☐ Black-shouldered Spinyleg ☐ -
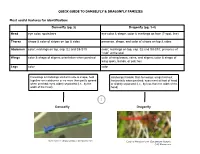
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Microspectrophotometry of Arthropod Visual Screening Pigments
Microspectrophotometry of Arthropod Visual Screening Pigments G. K. STROTHER and A.J. CASELLA From the Departments of Biophysics and Physics, The Pennsylvania State University, University Park, Pennsylvania 16802. Dr. Casella's present address is the Department of Physics, Jacksonville University, Jacksonville, Florida 32211. ABSTRACT Absorption spectra of visual screening pigments obtained in vitro with a microspectrophotometer using frozen sections are given for the insects Musca domestica, Phormia regina, Libellula luctuosa, Apis mellifera (worker honeybee only), Drosophila melanogaster (wild type only) and the arachnids Lycosa bal- timoriana and Lycosa miami. The spectral range covered is 260-700 nm for Lycosa and Drosophila and 310-700 nm for the remainder of the arthropods. A complete description of the instrumentation is given. For the flies, Phormia and Musca, light absorption by the yellow and red pigments is high from 310 to about 610 nm. This implies that for these insects there should be no wavelength shift in electroretinogram (ERG) results due to light leakage among neighboring om- matidia for this wavelength range. The same comment applies to Calliphora erythrocephala, which is known to have similar screening pigments. For some of the insects studied a close correspondence is noted between screening pigment absorption spectra and spectral sensitivity curves for individual photoreceptors, available in the literature. In some cases the screening pigment absorption spectra can be related to chemical extraction results, with the general observa- tion that some of the in vitro absorption peaks are shifted to the red. The Lycosa, Apis, and Libellula dark red pigments absorb strongly over a wide spectral range and therefore prevent chemical identification. -

SPECIES FACT SHEET Scientific Name: Erpetogomphus Compositus
SPECIES FACT SHEET Scientific Name : Erpetogomphus compositus (Hagen in Selys1858) Common Name : White-belted Ringtail Phylum: Arthropoda Class: Insecta Order: Odonata Suborder: Anisoptera Family: Gomphidae (clubtails) Conservation Status : Global Status (1990): G5 Rounded Global Status: G5 - Secure National Status: N5 State Statuses- Arizona (SNR), California (SNR), Idaho (SNR), Nevada (SNR), New Mexico (SNR), Oregon (SNR) , Texas (SNR), Wyoming (SNR). Utah ranks the species as SH (Possible extirpated, historical), and in Washington it is ranked as S1 (Critically imperiled because of extreme rarity or because it is somehow especially vulnerable to extinction or extirpation). (NatureServe 2008) Technical Description : Adult: Characteristic of the family Gomphidae, this species has small, widely separated eyes and enlarged posterior abdominal segments (often less apparent on females). The conspicuously pale-ringed abdomen and pale green thorax with four distinct dark stripes are diagnostic for this species (Paulson 1999). The thorax is whitish between one pair of stripes (Paulson 2007a). The wings are clear with a slight yellowing at their bases (Abbot 2007). Total length: 46-55 mm (1.8-2.2 in.); abdomen: 31-39 mm (1.2-1.5 in.); hindwing: 26-32 mm (1-1.3 in.). Additional descriptive information for the adult can be found at OdonataCentral: http://www.odonatacentral.org/index.php/FieldGuideAction.get/id/46076 (last accessed 5 Oct. 2008). Immature: Erpetogomphus in the Pacific Northwest can be identified by the following traits: prementum and palpal lobes flat (as opposed to cup-shaped), antennae 4-segmented, wing pads divergent, labium wide (maximum width more than half maximum width of head across eyes), tips of cerci extending at least 0.9 times (as opposed to 0.75 times) the distance to the tip of epiproct (Tennessen 2007). -

1 Common Dragonflies and Damselflies of the Chicago Region
WEB V ERSION Odonata of Northeastern Illinois, USA 1 Common Dragonflies and Damselflies of the Chicago Region Volunteer Stewardship Network – Chicago Wilderness Produced by: John & Jane Balaban, Jennie Kluse & Robin Foster, with assistance of Laurel Ross and support from the Gordon & Betty Moore Foundation. Photos © John & Jane Balaban; [[email protected]] North Branch Restoration Project, with additions by © Thomas Murray (27, 32) and © Vincent Hickey (30). © Environmental & Conservation Programs, The Field Museum, Chicago, IL 60605 USA. [http://www.fmnh.org/chicagoguides/]. Chicago Wilderness Guide #1 version 2 (4/2006) RESOURCES: LIBELLULIDAE - Skimmers Drangonflies of Indiana by J. R. Curry. Large, showy, frequently seen Indiana Academy of Science. 2001. ISBN: 1-883362-11-3 resting on or flying low over Beginner’s Guide to Dragonflies by Nikula and Sones vegetation. Often hunt from a perch with D. and L. Stokes. Little, Brown, and Company. 2002. ISBN: 0-316-81679-5 like Kingbirds. Also includes our Damselflies of the Northeast by E. Lam. Biodiversity smallest dragonflies (Nannothemis Books. 2004. ISBN: 0-9754015-0-5 Damselflies of the North Woods by B. DuBois. and Perithemis) and the ubiquitous Kollath-Stensaas Pub. 2005. ISBN: 0-9673793-7-7 Meadowhawks. http://bugguide.ent.iastate.edu/node/view/191/bgimage 1 Sympetrum rubicundulum / http://cirrusimage.com/dragonflies.htm Ruby Meadowhawk: male and female mating in http://wisconsinbutterflies.org/damselflies/ “wheel” position. 34-38mm 2 Sympetrum obtrusum 3 Sympetrum vicinum 4 Sympetrum semicinctum White-faced Meadowhawk: white face. 32-36mm Yellow-legged Meadowhawk: yellow legs. 30-36mm Band-winged Meadowhawk: half amber wings. 26-38mm Above species are medium-sized and common. -

New State Record and Notable Range Extension for Libellula Semifasciata (Odonata: Libellulidae)
The Great Lakes Entomologist Volume 43 Numbers 1 - 4 - 2010 Numbers 1 - 4 - 2010 Article 8 April 2010 New State Record and Notable Range Extension for Libellula Semifasciata (Odonata: Libellulidae) Ryan D. Rasmussen Muscatine Soil and Water Conservation District Joshua G. Otten Joseph W. Dixon United States Department of Agriculture Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Rasmussen, Ryan D.; Otten, Joshua G.; and Dixon, Joseph W. 2010. "New State Record and Notable Range Extension for Libellula Semifasciata (Odonata: Libellulidae)," The Great Lakes Entomologist, vol 43 (1) Available at: https://scholar.valpo.edu/tgle/vol43/iss1/8 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Rasmussen et al.: New State Record and Notable Range Extension for <i>Libellula Sem 2010 THE GREAT LAKES ENTOMOLOGIST 91 New State Record and Notable Range Extension for Libellula semifasciata (Odonata: Libellulidae) Ryan D. Rasmussen1, Joshua G. Otten2 and Joseph W. Dixon3 Abstract The painted skimmer, Libellula semifasciata Burmeister (Odonata: Libel- lulidae), is an eastern species of dragonfly that has never been documented in Iowa. In this note we report two observations and the collection of a voucher for this species in southeast Iowa in the last three years. Based on other records of this species, including those from neighboring states and more northerly latitudes, we propose that these observations are evidence of a range extension. -

Libellula Flavida (Odonata: Libellulidae), a Dragonfly New to Ohio
The Great Lakes Entomologist Volume 33 Numbers 3 & 4 - Fall/Winter 2000 Numbers 3 & Article 6 4 - Fall/Winter 2000 October 2000 Libellula Flavida (Odonata: Libellulidae), a Dragonfly New ot Ohio Tom D. Schultz Denison University Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Schultz, Tom D. 2000. "Libellula Flavida (Odonata: Libellulidae), a Dragonfly New ot Ohio," The Great Lakes Entomologist, vol 33 (3) Available at: https://scholar.valpo.edu/tgle/vol33/iss3/6 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Schultz: <i>Libellula Flavida</i> (Odonata: Libellulidae), a Dragonfly New 2000 THE GREAT LAKES ENTOMOLOGIST 205 LlBELLULA FLAVIDA (ODONATA: UBEllUUDAEj, A DRAGONFLY NEW TO OHIO Tom D. Schultzl ABSTRACT Libellula flavida, a "videspread but uncommon dragonfly of southeastern and south central North America, is now recorded from Ohio. A breeding pop ulation was discovered in an acidic fen on the site of a sandstone quarry in southern Ohio. Libellula flavida Rambur (Odonata: Libellulidae), the Yellow-sided Skim mer, is a medium-sized libellulid dragonfly with pale yellow sclerites on the sides of the adult pterothorax. Little has been written about this species, but records indicate a range extending from the mid-Atlantic coast, across the south, to the south-central plains states (Needham et al. 2000). -

Widow Skimmer
Widow Skimmer Dragonflies of N. Va. - Kevin Munroe, 2012 Flight Record: Widow Skimmer (Libellula luctuosa) – 1.8”, 42-50 mm (5/12- 10/02) Peaks July-August. Common Habitat: Vegetated pond, lake and marsh edges. Also M meadows. Males have unique combo of First M 1 dark & 1 light Glance: blotch per wing Medium. Long, black & white wings, with blue body (male), black blotched wings with yellow & brown body (female). Compare: F Common Whitetail, Females have Twelve- F one large dark spotted blotch per wing Skimmer Notes from the field – Widow Skimmer: The bold, contrasting colors on the wings of the male Widow Skimmer make this one of the most recognizable dragonflies in our area. Those wings give it an almost butterfly-like appearance, and one wonders at first if an acrobatic swallowtail hasn’t just visited the pond edge. As you can see in several of these photos, their beautiful wings are often torn and tattered, more so than other species it seems. They’re a member of the King Skimmer genus, so perhaps it’s their intensely territorial nature that leads to so many injured wings. The individual in the upper photo is missing almost an entire rear wing, but was still able to fly faster than this dragonfly geek could run. If you’re looking for Widows, check the vege- tated edges of your local pond or marsh any sunny day, June-September. They prefer tall plants for perching, so be sure to allow the native grasses and wildflowers to grow large and thick around your neighborhood water- ways. -

Dragonflies and Damselflies of Southeastern New York
Dragonflies and Damselflies of Southeastern New York Alan & Della Wells Odonata North America 511 species New Jersey 182 species New York 185 species Odonata Damselflies (Zygoptera) • At rest, wings meeting above body or partly extended • Front and hind wings similar in size and shape • Eyes separated by more than their own width • Generally, weak fliers Dragonflies (Anisoptera) • At rest, wings held horizontally • Hind wing considerably wider at base than front wing • Eyes meeting mid-dorsally or closely spaced • Generally, strong fliers Damselflies North America 161 species New Jersey 54 species New York 58 species Damselflies Broad-winged Damsels Spreadwings Pond Damsels Broad-winged Damsels Calopterygidae • Wings broad at base • Wings held together at rest • Wings with black, brown, amber or red • 6 NY species Ebony Jewelwing, Calopteryx maculata Ebony Jewelwing, Calopteryx maculata Spreadwings Lestidae • Wings narrow at base • Wings spread at rest • Wings clear or slightly tinted • Stigmas long, length > 2x width • 11 NY species Southern Spreadwing, Lestes australis Slender Spreadwing, Lestes rectangularis Sweetflag Spreadwing, Lestes forcipatus Swamp Spreadwing, Lestes vigilax Amber-winged Spreadwing ???, Lestes eurinus Pond Damsel Coenagrionidae • Wings narrow at base • Wings usually held together at rest • Wings clear or slightly tinted • Stigmas short, length width • 41 NY species Pond Damsels Common Name Genus NY Bluets (American) Enallagma 24 Dancers Argia 6 Forktails Ischnura 6 Sprites Nehalennia 3 Aurora Damsel Chromagrion 1 Red Damsel Amphiagrion 1 Bluets (Eurasian) Coenagrion 1 Tech Note: How to tell Bluets from Dancers... Look for, Variable (Violet) Dancer, Argia fumipennis Variable (Violet) Dancer, Argia fumipennis Powdered Dancer, Argia moesta Powdered Dancer, Argia moesta Blue-fronted Dancer, Argia apicalis Dusky Dancer, Argia translata Blue-tipped Dancer, Argia tibialis (S1) Blue-tipped Dancer, Argia tibialis (Blue Form) (S1) Tech Note: Distinquishing Bluets and related damsels.. -

Common Odonta—Dragonflies and Damselflies
Dragonfly identification guide University of Houston Coastal Center Scott Clark - June, 2015 Photography by Scott Clark © 2015, all rights reserved. Contact: [email protected], 832.723.8339 Quick reference Widow skimmer, p. 11 Halloween pennant, p. 3 Eastern pondhawk, p. 7 Swamp darner, p. 15 Common whitetail , p. 12 Calico pennant, p. 4 Great Blue skimmer, p. 8 Marl pennant, p. 16 Needham’s skimmer, p. 5 Four-spotted pennant, p. 13 Blue dasher, p. 9 Eastern amberwing, p. 17 Broad-striped Red-tailed pennant, p. 6 Seaside dragonlet, p. 10 Black saddlebags, p. 14 foreceptail, p. 18 Halloween pennant (male) (female) Celithemis eponina Season: April - October Length: 36-42 mm Observation: June 11, 2015, 1:15 PM Location: Perched on rushes at pond Features: Alternating black and orange bands on wings; female same pattern but yellow Similar species: Banded and calico pennants (mating pair) 3 Calico pennant (male) (female) Celithemis elisa Season: April - September Length: 29-34 mm Observation: June 10, 2015, 12:45 PM Location: On forbs adjacent to pond Features: Checkerboard abdomen, spots on forewings, large patch on base of hind wing and black wing tips; female yellow. Similar species: Similar pennants either have clear wings or different markings 4 Needham’s skimmer (male) (female) Libellula needhami Season: May – November Length: 53-56 mm Observation: June 10, 2015, 12:15 PM Location: Perched on sedge at pond Features: Red-orange with orange on front of (male) wing, dark stripe on abdomen, brown legs; female same pattern but yellow with