Microspectrophotometry of Arthropod Visual Screening Pigments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dragonflies September 2008
Learning Laguna Field Notes: Dragonflies September 2008 This edition is based on a Continuing Education event taught by Kathy & Dave Biggs. Dragonfly is a type of insect belonging to the order Odonata. As such, they have 3 prominent body parts—a head, a thorax to which the wings (in this case 4) and 6 legs are attached, and an abdomen. Their front and rear wings are not linked together, but can be operated independently. It is characterized by large multifaceted eyes, two pairs of strong, transparent wings, and an elongated body. Dragonflies typi- cally eat gnats, mosquitoes, and other small insects like flies, bees, and even butterflies. They are valued as predators, since they help control populations of potentially harmful insects and “pests.” Eight Spotted Skimmer - male (Libellula forensis) Mark A. Chappell Some Dragonfly “Wow” Facts http://faculty.ucr.edu/~chappell/INW/arthropods/8spot1.jpg • Because of their compound eyes, they can see in all directions and can see flickers of movement 15 times better than humans. • Predators like birds, especially the Black Phoebe, will eat them, but will drop the wings as they have barbs • In most dragonfly species, the eyes touch • Their legs are studded with spikes and they capture prey by clasping it in their legs. • Dragonflies usually attack prey from below • Like most bird species, males are more colorful that females • In the United States dragonflies and damselflies are sought out as a hobby similar to birding and butterflying, known as oding. Oding, from the dragonfly’s scientific order name: Odo- nata. • Dragonflies are survivors from before the dinosaur period. -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Checklist Dragonfly and Damselfly
To report sightings, contact: Natural Resources Coordinator 980-314-1119 www.parkandrec.com DRAGONFLY AND DAMSELFLY CHECKLIST Mecklenburg County, NC: 88 species Petaltails ☐ Swift River Crusier ☐ Autumn Meadowhawk ☐ Gray Petaltail (Tachopteryx thoreyi)*∆ (Macromia illinoiensis)*∆ (Sympetrum vicinum)*∆ ☐ Royal River Cruiser ☐ Carolina Saddlebags (Tramea carolina)*∆ Darners (Macromia taeniolata)*∆ ☐ Black Saddlebags (Tramea lacerata)*∆ ☐ Shadow Darner (Aeshna umbrosa) ☐ Common Green Darner (Anax junius)*∆ Emeralds Broad-winged Damsels ☐ Comet Darner (Anax longipes)*∆ ☐ Common Baskettail (Epitheca cynosura)*∆ ☐ Sparkling Jewelwing ☐ Springtime Darner (Basiaeschna janata)* ☐ Prince Baskettail (Epitheca princeps)*∆ (Calopteryx dimidiata)* ☐ Fawn Darner (Boyeria vinosa) ☐ Selys’ Sundragon (Helocordulia selysii) ☐ Ebony Jewelwing (Calopteryx maculata)*∆ ☐ Swamp Darner (Epiaeschna heros)*∆ ☐ Mocha Emerald (Somatochlora linearis)*∆ ☐ Smoky Rubyspot (Hetaerina titia) ☐ Taper-tailed Darner ☐ Clamp-tipped Emerald Spreadwings (Gomphaeschna antilope)*∆ (Somatochlora tenebrosa)*∆ ☐ Elegant Spreadwing (Lestes inaequalis)* ☐ Cyrano Darner Skimmers ☐ Southern Spreadwing (Lestes australis) (Nasiaeschna pentacantha)*∆ ☐ Four-spotted Pennant ☐ Amber-winged Spreadwing Clubtails (Brachymesia gravida)*∆ (Lestes eurinus)* ☐ Two-striped Forceptail ☐ Calico Pennant (Celithemis elisa)*∆ ☐ Slender Spreadwing (Lestes rectangularis)* (Aphylla williamsoni)*∆ ☐ Halloween Pennant (Celithemis eponina)*∆ ☐ Swamp Spreadwing (Lestes vigilax) ☐ Black-shouldered Spinyleg ☐ -
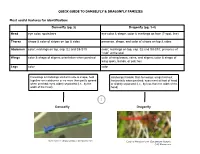
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Dragonflies of A( Nisoptera) Arkansas George L
Journal of the Arkansas Academy of Science Volume 31 Article 17 1977 Dragonflies of A( nisoptera) Arkansas George L. Harp Arkansas State University John D. Rickett University of Arkansas at Little Rock Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Entomology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Harp, George L. and Rickett, John D. (1977) "Dragonflies of (Anisoptera) Arkansas," Journal of the Arkansas Academy of Science: Vol. 31 , Article 17. Available at: http://scholarworks.uark.edu/jaas/vol31/iss1/17 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 31 [1977], Art. 17 The Dragonflies (Anisoptera) of Arkansas GEORGE L.HARP Division of Biological Sciences, Arkansas State University State University, Arkansas 72467 JOHND. RICKETT Department of Biology, University of Arkansas at LittleRock LittleRock, Arkansas 72204 ABSTRACT Previous publications have recorded 69 species of dragonflies for Arkansas. Three of these are deleted, but state records for 21 new species are reported herein, bringing the list to 87 species. -

Phylogeny of the Higher Libelluloidea (Anisoptera: Odonata): an Exploration of the Most Speciose Superfamily of Dragonflies
Molecular Phylogenetics and Evolution 45 (2007) 289–310 www.elsevier.com/locate/ympev Phylogeny of the higher Libelluloidea (Anisoptera: Odonata): An exploration of the most speciose superfamily of dragonflies Jessica Ware a,*, Michael May a, Karl Kjer b a Department of Entomology, Rutgers University, 93 Lipman Drive, New Brunswick, NJ 08901, USA b Department of Ecology, Evolution and Natural Resources, Rutgers University, 14 College Farm Road, New Brunswick, NJ 08901, USA Received 8 December 2006; revised 8 May 2007; accepted 21 May 2007 Available online 4 July 2007 Abstract Although libelluloid dragonflies are diverse, numerous, and commonly observed and studied, their phylogenetic history is uncertain. Over 150 years of taxonomic study of Libelluloidea Rambur, 1842, beginning with Hagen (1840), [Rambur, M.P., 1842. Neuropteres. Histoire naturelle des Insectes, Paris, pp. 534; Hagen, H., 1840. Synonymia Libellularum Europaearum. Dissertation inaugularis quam consensu et auctoritate gratiosi medicorum ordinis in academia albertina ad summos in medicina et chirurgia honores.] and Selys (1850), [de Selys Longchamps, E., 1850. Revue des Odonates ou Libellules d’Europe [avec la collaboration de H.A. Hagen]. Muquardt, Brux- elles; Leipzig, 1–408.], has failed to produce a consensus about family and subfamily relationships. The present study provides a well- substantiated phylogeny of the Libelluloidea generated from gene fragments of two independent genes, the 16S and 28S ribosomal RNA (rRNA), and using models that take into account non-independence of correlated rRNA sites. Ninety-three ingroup taxa and six outgroup taxa were amplified for the 28S fragment; 78 ingroup taxa and five outgroup taxa were amplified for the 16S fragment. -

SPECIES FACT SHEET Scientific Name: Erpetogomphus Compositus
SPECIES FACT SHEET Scientific Name : Erpetogomphus compositus (Hagen in Selys1858) Common Name : White-belted Ringtail Phylum: Arthropoda Class: Insecta Order: Odonata Suborder: Anisoptera Family: Gomphidae (clubtails) Conservation Status : Global Status (1990): G5 Rounded Global Status: G5 - Secure National Status: N5 State Statuses- Arizona (SNR), California (SNR), Idaho (SNR), Nevada (SNR), New Mexico (SNR), Oregon (SNR) , Texas (SNR), Wyoming (SNR). Utah ranks the species as SH (Possible extirpated, historical), and in Washington it is ranked as S1 (Critically imperiled because of extreme rarity or because it is somehow especially vulnerable to extinction or extirpation). (NatureServe 2008) Technical Description : Adult: Characteristic of the family Gomphidae, this species has small, widely separated eyes and enlarged posterior abdominal segments (often less apparent on females). The conspicuously pale-ringed abdomen and pale green thorax with four distinct dark stripes are diagnostic for this species (Paulson 1999). The thorax is whitish between one pair of stripes (Paulson 2007a). The wings are clear with a slight yellowing at their bases (Abbot 2007). Total length: 46-55 mm (1.8-2.2 in.); abdomen: 31-39 mm (1.2-1.5 in.); hindwing: 26-32 mm (1-1.3 in.). Additional descriptive information for the adult can be found at OdonataCentral: http://www.odonatacentral.org/index.php/FieldGuideAction.get/id/46076 (last accessed 5 Oct. 2008). Immature: Erpetogomphus in the Pacific Northwest can be identified by the following traits: prementum and palpal lobes flat (as opposed to cup-shaped), antennae 4-segmented, wing pads divergent, labium wide (maximum width more than half maximum width of head across eyes), tips of cerci extending at least 0.9 times (as opposed to 0.75 times) the distance to the tip of epiproct (Tennessen 2007). -

Prioritizing Odonata for Conservation Action in the Northeastern USA
APPLIED ODONATOLOGY Prioritizing Odonata for conservation action in the northeastern USA Erin L. White1,4, Pamela D. Hunt2,5, Matthew D. Schlesinger1,6, Jeffrey D. Corser1,7, and Phillip G. deMaynadier3,8 1New York Natural Heritage Program, State University of New York College of Environmental Science and Forestry, 625 Broadway 5th Floor, Albany, New York 12233-4757 USA 2Audubon Society of New Hampshire, 84 Silk Farm Road, Concord, New Hampshire 03301 USA 3Maine Department of Inland Fisheries and Wildlife, 650 State Street, Bangor, Maine 04401 USA Abstract: Odonata are valuable biological indicators of freshwater ecosystem integrity and climate change, and the northeastern USA (Virginia to Maine) is a hotspot of odonate diversity and a region of historical and grow- ing threats to freshwater ecosystems. This duality highlights the urgency of developing a comprehensive conser- vation assessment of the region’s 228 resident odonate species. We offer a prioritization framework modified from NatureServe’s method for assessing conservation status ranks by assigning a single regional vulnerability metric (R-rank) reflecting each species’ degree of relative extinction risk in the northeastern USA. We calculated the R-rank based on 3 rarity factors (range extent, area of occupancy, and habitat specificity), 1 threat factor (vulnerability of occupied habitats), and 1 trend factor (relative change in range size). We combine this R-rank with the degree of endemicity (% of the species’ USA and Canadian range that falls within the region) as a proxy for regional responsibility, thereby deriving a list of species of combined vulnerability and regional management responsibility. Overall, 18% of the region’s odonate fauna is imperiled (R1 and R2), and peatlands, low-gradient streams and seeps, high-gradient headwaters, and larger rivers that harbor a disproportionate number of these species should be considered as priority habitat types for conservation. -

1 Common Dragonflies and Damselflies of the Chicago Region
WEB V ERSION Odonata of Northeastern Illinois, USA 1 Common Dragonflies and Damselflies of the Chicago Region Volunteer Stewardship Network – Chicago Wilderness Produced by: John & Jane Balaban, Jennie Kluse & Robin Foster, with assistance of Laurel Ross and support from the Gordon & Betty Moore Foundation. Photos © John & Jane Balaban; [[email protected]] North Branch Restoration Project, with additions by © Thomas Murray (27, 32) and © Vincent Hickey (30). © Environmental & Conservation Programs, The Field Museum, Chicago, IL 60605 USA. [http://www.fmnh.org/chicagoguides/]. Chicago Wilderness Guide #1 version 2 (4/2006) RESOURCES: LIBELLULIDAE - Skimmers Drangonflies of Indiana by J. R. Curry. Large, showy, frequently seen Indiana Academy of Science. 2001. ISBN: 1-883362-11-3 resting on or flying low over Beginner’s Guide to Dragonflies by Nikula and Sones vegetation. Often hunt from a perch with D. and L. Stokes. Little, Brown, and Company. 2002. ISBN: 0-316-81679-5 like Kingbirds. Also includes our Damselflies of the Northeast by E. Lam. Biodiversity smallest dragonflies (Nannothemis Books. 2004. ISBN: 0-9754015-0-5 Damselflies of the North Woods by B. DuBois. and Perithemis) and the ubiquitous Kollath-Stensaas Pub. 2005. ISBN: 0-9673793-7-7 Meadowhawks. http://bugguide.ent.iastate.edu/node/view/191/bgimage 1 Sympetrum rubicundulum / http://cirrusimage.com/dragonflies.htm Ruby Meadowhawk: male and female mating in http://wisconsinbutterflies.org/damselflies/ “wheel” position. 34-38mm 2 Sympetrum obtrusum 3 Sympetrum vicinum 4 Sympetrum semicinctum White-faced Meadowhawk: white face. 32-36mm Yellow-legged Meadowhawk: yellow legs. 30-36mm Band-winged Meadowhawk: half amber wings. 26-38mm Above species are medium-sized and common. -

Preliminary Survey and Inventory of Odonates of the Primitive Texas Big Thicket-With Notes on Their Distribution During the 2010-2011 Texas Drought
Survey of Big Thicket Odonates: Preliminary Survey and Inventory of Odonates of the Primitive Texas Big Thicket-with notes on their distribution during the 2010-2011 Texas Drought *Autumn J. Smith-Herron and **Tamara J. Cook *Texas Research Institute for Environmental Studies; **Department of Biological Sciences Sam Houston State University, Huntsville, Texas 77341-2506 *[email protected] Abstract. — As part of an ongoing survey of odonate diversity in the Big Thicket and Primitive Big Thicket regions of east-central Texas, U.S.A. (including some collections in west and south Texas), we made some interesting observations about odonate populations during the drought year 2010-2011in comparison to previous (non-drought) years. Our current research not only documents odonate diversity, but the gregarine parasite communities within (parasite communities nested within odonate communities). In part, this has allowed us to document trends in odonate species success over several years of field collections as well as discover/describe new parasite species. The most interesting trend however is noted during the 2010-2011 drought across Texas. In addition, during these surveys, we have discovered and documented several new odonate county records as well as one state record. Introduction. — During a year-long survey and inventory of Odonata from the Big Thicket National Preserve and surrounding areas of southeast Texas, we noted a significant decline in diversity within certain groups of Zygoptera in comparison to previous years. The onset of the 2010-2011 collecting season was initiated as a result of funding provided through the Big Thicket Association, and served to document the odonate fauna from the Big Thicket National Preserve (and Primitive Big Thicket areas). -

New State Record and Notable Range Extension for Libellula Semifasciata (Odonata: Libellulidae)
The Great Lakes Entomologist Volume 43 Numbers 1 - 4 - 2010 Numbers 1 - 4 - 2010 Article 8 April 2010 New State Record and Notable Range Extension for Libellula Semifasciata (Odonata: Libellulidae) Ryan D. Rasmussen Muscatine Soil and Water Conservation District Joshua G. Otten Joseph W. Dixon United States Department of Agriculture Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Rasmussen, Ryan D.; Otten, Joshua G.; and Dixon, Joseph W. 2010. "New State Record and Notable Range Extension for Libellula Semifasciata (Odonata: Libellulidae)," The Great Lakes Entomologist, vol 43 (1) Available at: https://scholar.valpo.edu/tgle/vol43/iss1/8 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Rasmussen et al.: New State Record and Notable Range Extension for <i>Libellula Sem 2010 THE GREAT LAKES ENTOMOLOGIST 91 New State Record and Notable Range Extension for Libellula semifasciata (Odonata: Libellulidae) Ryan D. Rasmussen1, Joshua G. Otten2 and Joseph W. Dixon3 Abstract The painted skimmer, Libellula semifasciata Burmeister (Odonata: Libel- lulidae), is an eastern species of dragonfly that has never been documented in Iowa. In this note we report two observations and the collection of a voucher for this species in southeast Iowa in the last three years. Based on other records of this species, including those from neighboring states and more northerly latitudes, we propose that these observations are evidence of a range extension. -

Inventory of Odonata (Dragonflies and Damselflies) at Gateway National Recreation Area
National Park Service U.S. Department of the Interior Natural Resource Program Center Inventory of Odonata (Dragonflies and Damselflies) at Gateway National Recreation Area Natural Resource Technical Report NPS/NCBN/NRTR—2010/296 ON THE COVER Swamp Darner (Epiaeschna heros ) at Gateway National Recreation Area . Photograph by: Jackie Sones. Inventory of Odonata (Dragonflies and Damselflies) at Gateway National Recreation Area Natural Resource Technical Report NPS/NCBN/NRTR—2010/296 Nina Briggs, Eric G. Schneider, Jackie Sones, Kristen Puryear Rhode Island Natural History P.O. Box 1858 Kingston, Rhode Island 02881 March 2010 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Technical Report Series is used to disseminate results of scientific studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service mission. The series provides contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. This report received informal peer review by subject-matter experts who were not directly involved in the collection, analysis, or reporting of the data.