The Paleoproterozoic Fossil Record: Implications for the Evolution of the Biosphere During Earth’S Middle-Age Emmanuelle J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oscillatoriales, Microcoleaceae), Nuevo Reporte Para El Perú
Montoya et al.: Diversidad fenotípica de la cianobacteria Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), nuevo reporte para el Perú Arnaldoa 24 (1): 369 - 382, 2017 ISSN: 1815-8242 (edición impresa) http://doi.org/10.22497/arnaldoa.241.24119 ISSN: 2413-3299 (edición online) Diversidad fenotípica de la cianobacteria Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), nuevo reporte para el Perú Phenotypic diversity of the cyanobacterium Pseudo- phormidium tenue (Oscillatoriales, Microcoleaceae), new record for Peru Haydee Montoya T., José Gómez C., Mauro Mariano A., Enoc Jara P., Egma Mayta H., Mario Benavente P. Museo de Historia Natural, Departamento de Simbiosis Vegetal, UNMSM. Av. Arenales 1256. Apartado 14-0434. Lima 14, PERÚ. Instituto de Investigación de Ciencias Biológicas, Facultad de CC. Biológicas, UNMSM [email protected], [email protected], [email protected] [email protected] 24 (1): Enero - Junio, 2017 369 Este es un artículo de acceso abierto bajo la licencia CC BY-NC 4.0: https://creativecommons.org/licenses/by-nc/4.0/ Montoya et al.: Diversidad fenotípica de la cianobacteria Pseudophormidium tenue (Oscillatoriales, Microcoleaceae), nuevo reporte para el Perú Recibido: 20-I-2017; Aceptado: 15-III-2017; Publicado: VI-2017; Edición online: 05-VI-2017 Resumen Los ecosistemas desérticos costeros tropicales están distribuidos ampliamente en el oeste de Sudamérica. No obstante las tierras áridas de esta región, la disponibilidad hídrica de la humedad proveniente de las neblinas a nivel del océano Pacífico acarreadas hacia las colinas (lomas) y las garúas anuales invernales fluctuantes favorecen el desarrollo de comunidades cianobacteriales extremas. El área de evaluación fue las Lomas de Pachacámac, al sur de Lima, y las colecciones cianobacteriales estándar (costras, biofilms o matas terrestres) fueron realizadas irregularmente en 1995 y 2012. -

Ediacaran) of Earth – Nature’S Experiments
The Early Animals (Ediacaran) of Earth – Nature’s Experiments Donald Baumgartner Medical Entomologist, Biologist, and Fossil Enthusiast Presentation before Chicago Rocks and Mineral Society May 10, 2014 Illinois Famous for Pennsylvanian Fossils 3 In the Beginning: The Big Bang . Earth formed 4.6 billion years ago Fossil Record Order 95% of higher taxa: Random plant divisions domains & kingdoms Cambrian Atdabanian Fauna Vendian Tommotian Fauna Ediacaran Fauna protists Proterozoic algae McConnell (Baptist)College Pre C - Fossil Order Archaean bacteria Source: Truett Kurt Wise The First Cells . 3.8 billion years ago, oxygen levels in atmosphere and seas were low • Early prokaryotic cells probably were anaerobic • Stromatolites . Divergence separated bacteria from ancestors of archaeans and eukaryotes Stromatolites Dominated the Earth Stromatolites of cyanobacteria ruled the Earth from 3.8 b.y. to 600 m. [2.5 b.y.]. Believed that Earth glaciations are correlated with great demise of stromatolites world-wide. 8 The Oxygen Atmosphere . Cyanobacteria evolved an oxygen-releasing, noncyclic pathway of photosynthesis • Changed Earth’s atmosphere . Increased oxygen favored aerobic respiration Early Multi-Cellular Life Was Born Eosphaera & Kakabekia at 2 b.y in Canada Gunflint Chert 11 Earliest Multi-Cellular Metazoan Life (1) Alga Eukaryote Grypania of MI at 1.85 b.y. MI fossil outcrop 12 Earliest Multi-Cellular Metazoan Life (2) Beads Horodyskia of MT and Aust. at 1.5 b.y. thought to be algae 13 Source: Fedonkin et al. 2007 Rise of Animals Tappania Fungus at 1.5 b.y Described now from China, Russia, Canada, India, & Australia 14 Earliest Multi-Cellular Metazoan Animals (3) Worm-like Parmia of N.E. -

Algal Stromatolites in the Willow River Member of the Lower Ordovician Shakopee Formation Near Chatfield, Minnesota, USA
The Compass: Earth Science Journal of Sigma Gamma Epsilon Volume 84 Issue 1 Article 6 1-6-2012 Algal Stromatolites in the Willow River Member of the Lower Ordovician Shakopee Formation near Chatfield, Minnesota, USA Sophia L. May College of St. Benedict / St. John's University, [email protected] Larry E. Davis College of St. Benedict / St. John's University, [email protected] David G. Brown College of St. Benedict / St. John's University, [email protected] Follow this and additional works at: https://digitalcommons.csbsju.edu/compass Part of the Paleontology Commons Recommended Citation May, Sophia L.; Davis, Larry E.; and Brown, David G. (2012) "Algal Stromatolites in the Willow River Member of the Lower Ordovician Shakopee Formation near Chatfield, Minnesota, USA," The Compass: Earth Science Journal of Sigma Gamma Epsilon: Vol. 84: Iss. 1, Article 6. Available at: https://digitalcommons.csbsju.edu/compass/vol84/iss1/6 This Article is brought to you for free and open access by DigitalCommons@CSB/SJU. It has been accepted for inclusion in The Compass: Earth Science Journal of Sigma Gamma Epsilon by an authorized editor of DigitalCommons@CSB/SJU. For more information, please contact [email protected]. ON THE OUTCROP Algal Stromatolites in the Willow River Member of the Lower Ordovician Shakopee Formation near Chatfield, Minnesota, USA Sophia L. May, Larry E. Davis, and David G. Brown Department of Biology College of Saint Benedict/Saint John’s University Collegeville, Minnesota, 56321 USA [email protected] LOCATION From the intersection of (Olmsted) Co. Hwy 2 and U.S. 52 Rochester I-90 (Main Street) in Chatfield, MN drive N south-southeast on U.S. -

Planktothrix Agardhii É a Mais Comum
Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters Catarina Isabel Prata Pereira Leitão Churro Doutoramento em Biologia Departamento Biologia 2015 Orientador Vitor Manuel de Oliveira e Vasconcelos, Professor Catedrático Faculdade de Ciências iv FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters The research presented in this thesis was supported by the Portuguese Foundation for Science and Technology (FCT, I.P.) national funds through the project PPCDT/AMB/67075/2006 and through the individual Ph.D. research grant SFRH/BD65706/2009 to Catarina Churro co-funded by the European Social Fund (Fundo Social Europeu, FSE), through Programa Operacional Potencial Humano – Quadro de Referência Estratégico Nacional (POPH – QREN) and Foundation for Science and Technology (FCT). The research was performed in the host institutions: National Institute of Health Dr. Ricardo Jorge (INSA, I.P.), Lisboa; Interdisciplinary Centre of Marine and Environmental Research (CIIMAR), Porto and Centre for Microbial Resources (CREM - FCT/UNL), Caparica that provided the laboratories, materials, regents, equipment’s and logistics to perform the experiments. v FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters vi FCUP Accessing Planktothrix species diversity and associated toxins using quantitative real-time PCR in natural waters ACKNOWLEDGMENTS I would like to express my gratitude to my supervisor Professor Vitor Vasconcelos for accepting to embark in this research and supervising this project and without whom this work would not be possible. I am also greatly thankful to my co-supervisor Elisabete Valério for the encouragement in pursuing a graduate program and for accompanying me all the way through it. -

Appendix: Economic Geology: Exploration for Coal, Oil and Minerals
Downloaded from http://mem.lyellcollection.org/ by guest on October 1, 2021 PART 4 Appendix: Economic geology: exploration for coal, oil and Glossary of stratigraphic names, 463 minerals, 449 References, 477 Index of place names, 455 General Index, 515 Alkahornet, a distinctive landmark on the northwest, entrance to Isfjorden, is formed of early Varanger carbonates. The view is from Trygghamna ('Safe Harbour') with CSE motorboats Salterella and Collenia by the shore, with good anchorage and easy access inland. Photo M. J. Hambrey, CSE (SP. 1561). Routine journeys to the fjords of north Spitsbergen and Nordaustlandet pass by the rocky coastline of northwest Spitsbergen. Here is a view of Smeerenburgbreen from Smeerenburgfjordenwhich affords some shelter being protected by outer islands. On one of these was Smeerenburg, the principal base for early whaling, hence the Dutch name for 'blubber town'. Photo N. I. Cox, CSE 1989. Downloaded from http://mem.lyellcollection.org/ by guest on October 1, 2021 The CSE motorboat Salterella in Liefdefjorden looking north towards Erikbreen with largely Devonian rocks in the background unconformably on metamorphic Proterozoic to the left. Photo P. W. Web, CSE 1989. Access to cliffs and a glacier route (up Hannabreen) often necessitates crossing blocky talus (here Devonian in foreground) and then possibly a pleasanter route up the moraine on to hard glacier ice. Moraine generally affords a useful introduction to the rocks to be traversed along the glacial margin. The dots in the sky are geese training their young to fly in V formation for their migration back to the UK at the end of the summer. -

Microcoleus Pseudautumnalis Sp. Nov. (Cyanobacteria, Oscillatoriales) Producing 2-Methylisoborneol
Bull. Natl. Mus. Nat. Sci., Ser. B, 45(3), pp. 93–101, August 22, 2019 Microcoleus pseudautumnalis sp. nov. (Cyanobacteria, Oscillatoriales) producing 2-methylisoborneol Yuko Niiyama* and Akihiro Tuji Department of Botany, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan * E-mail: [email protected] (Received 13 May 2019; accepted 26 June 2019) Abstract A new species, Microcoleus pseudautumnalis, producing both 2-methylisoborneol (2-MIB) and geosmin is described. We have conducted a systematic study of a bad-smelling, 2-MIB producing planktic Pseudanabaena species in Japan and described four new species (P. foetida, P. subfoetida, P. cinerea, and P. yagii). In the course of this study, we found another kind of filamentous cyanobacteria with a bad smell in a plankton sample collected from a pond in Japan. The morphology of M. pseudautumnalis resembles that of M. autumnalis (Trevisan ex Gomont) Strunecký, Komárek et Johansen (basionym: Phormidium autumnale Trevisan ex Gomont). The sheath is thin and always contains only one trichome. Trichomes are immotile, gray- ish-green, not constricted at the cross-walls, not attenuated or attenuated towards the ends with truncated or capitated apical cells, and sometimes with calyptrae that are relatively wider (6.9– 7.6 μm) than those of M. autumnalis. The phylogeny of the 16S rRNA gene of M. pseudautumnalis revealed that it is in the clade of the genus Microcoleus and contains an 11-bp insert. Microcoleus autumnalis s. str. is said to lack this insert. Microcoleus pseudautumnalis has four kinds of 2-MIB genes, and the phylogeny of this taxon is different from those of Pseudanabaena sp. -

Cyanobacterial Evolution During the Precambrian
International Journal of Astrobiology 15 (3): 187–204 (2016) doi:10.1017/S1473550415000579 © Cambridge University Press 2016 This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited. Cyanobacterial evolution during the Precambrian Bettina E. Schirrmeister1, Patricia Sanchez-Baracaldo2 and David Wacey1,3 1School of Earth Sciences, University of Bristol, Wills Memorial Building, Queen’s Road, Bristol BS8 1RJ, UK e-mail: [email protected] 2School of Geographical Sciences, University of Bristol, University Road, Bristol BS8 1SS, UK 3Centre for Microscopy, Characterisation and Analysis, and ARC Centre of Excellence for Core to Crust Fluid Systems, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia Abstract: Life on Earth has existed for at least 3.5 billion years. Yet, relatively little is known of its evolution during the first two billion years, due to the scarceness and generally poor preservation of fossilized biological material. Cyanobacteria, formerly known as blue green algae were among the first crown Eubacteria to evolve and for more than 2.5 billion years they have strongly influenced Earth’s biosphere. Being the only organism where oxygenic photosynthesis has originated, they have oxygenated Earth’s atmosphere and hydrosphere, triggered the evolution of plants –being ancestral to chloroplasts– and enabled the evolution of complex life based on aerobic respiration. Having such a strong impact on early life, one might expect that the evolutionary success of this group may also have triggered further biosphere changes during early Earth history. -

Thiomargarita Namibiensis Cells by Using Microelectrodes Heide N
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Nov. 2002, p. 5746–5749 Vol. 68, No. 11 0099-2240/02/$04.00ϩ0 DOI: 10.1128/AEM.68.11.5746–5749.2002 Copyright © 2002, American Society for Microbiology. All Rights Reserved. Uptake Rates of Oxygen and Sulfide Measured with Individual Thiomargarita namibiensis Cells by Using Microelectrodes Heide N. Schulz1,2* and Dirk de Beer1 Max Planck Institute for Marine Microbiology, D-28359 Bremen, Germany,1 and Section of Microbiology, University of California, Davis, Davis, California 956162 Received 25 March 2002/Accepted 31 July 2002 Gradients of oxygen and sulfide measured towards individual cells of the large nitrate-storing sulfur bacterium Thiomargarita namibiensis showed that in addition to nitrate oxygen is used for oxidation of sulfide. Stable gradients around the cells were found only if acetate was added to the medium at low concentrations. The sulfur bacterium Thiomargarita namibiensis is a close conclusions about their physiology by observing chemotactic relative of the filamentous sulfur bacteria of the genera Beg- behavior, as has been done successfully with Beggiatoa and giatoa and Thioploca. It was only recently discovered off the Thioploca filaments (5, 10). However, because of the large size Namibian coast in fluid sediments rich in organic matter and of Thiomargarita cells, they develop, around individual cells, sulfide (15). The large, spherical cells of Thiomargarita (diam- measurable gradients of oxygen and sulfide that can be used eter, 100 to 300 m) are held together in a chain by mucus that for calculating uptake rates of oxygen and sulfide. Thus, the surrounds each cell (Fig. 1). Most of the cell volume is taken physiological reactions of individual cells to changes in oxygen up by a central vacuole in which nitrate is stored at concen- and sulfide concentrations can be directly observed by observ- trations of up to 800 mM. -

GEOLOGY THEME STUDY Page 1
NATIONAL HISTORIC LANDMARKS Dr. Harry A. Butowsky GEOLOGY THEME STUDY Page 1 Geology National Historic Landmark Theme Study (Draft 1990) Introduction by Dr. Harry A. Butowsky Historian, History Division National Park Service, Washington, DC The Geology National Historic Landmark Theme Study represents the second phase of the National Park Service's thematic study of the history of American science. Phase one of this study, Astronomy and Astrophysics: A National Historic Landmark Theme Study was completed in l989. Subsequent phases of the science theme study will include the disciplines of biology, chemistry, mathematics, physics and other related sciences. The Science Theme Study is being completed by the National Historic Landmarks Survey of the National Park Service in compliance with the requirements of the Historic Sites Act of l935. The Historic Sites Act established "a national policy to preserve for public use historic sites, buildings and objects of national significance for the inspiration and benefit of the American people." Under the terms of the Act, the service is required to survey, study, protect, preserve, maintain, or operate nationally significant historic buildings, sites & objects. The National Historic Landmarks Survey of the National Park Service is charged with the responsibility of identifying America's nationally significant historic property. The survey meets this obligation through a comprehensive process involving thematic study of the facets of American History. In recent years, the survey has completed National Historic Landmark theme studies on topics as diverse as the American space program, World War II in the Pacific, the US Constitution, recreation in the United States and architecture in the National Parks. -

12.007 Geobiology Spring 2009
MIT OpenCourseWare http://ocw.mit.edu 12.007 Geobiology Spring 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Geobiology 2009 Lecture 10 The Antiquity of Life on Earth Homework #5 Topics (choose 1): Describe criteria for biogenicity in microscopic fossils. How do the oldest describes fossils compare? Use this to argue one side of the Brasier-Schopf debate OR What are stromatolites; where are they found and how are they formed? Articulate the two sides of the debate on antiquity and biogenicity. Up to 4 pages, including figures. Due 3/31/2009 Need to know • How C and S- isotopic data in rocks are informative about the advent and antiquity of biogeochemical cycles • Morphological remains and the antiquity of life; how do we weigh the evidence? • Indicators of changes in atmospheric pO2 • A general view of the course of oxygenation of the atm-ocean system Readings for this lecture Schopf J.W. et al., (2002) Laser Raman Imagery of Earth’s earliest fossils. Nature 416, 73. Brasier M.D. et al., (2002) Questioning the evidence for Earth’s oldest fossils. Nature 416, 76. Garcia-Ruiz J.M., Hyde S.T., Carnerup A. M. , Christy v, Van Kranendonk M. J. and Welham N. J. (2003) Self-Assembled Silica-Carbonate Structures and Detection of Ancient Microfossils Science 302, 1194-7. Hofmann, H.J., Grey, K., Hickman, A.H., and Thorpe, R. 1999. Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geological Society of America, Bulletin, v. 111 (8), p. -

Isolation and Identification of Denitrifying Bacteria and Its Growth and Metabolism Characteristics
2017 2nd International Conference on Environmental Science and Energy Engineering (ICESEE 2017) ISBN: 978-1-60595-417-2 Isolation and Identification of Denitrifying Bacteria and Its Growth and Metabolism Characteristics Xin-ran JIANG, Dian JIAO, Lei ZHANG and Li-na ZHENG College of Marine Technology and Environmental, Dalian Ocean University, Dalian 116023, China Keywords: Denitrification, Nitrate, Isolation and identification, Activity research. Abstract. A denitrifying bacterium DN20 was isolated from the biofilm after centrifugation in the aquaculture purification system of a aquaculture base, and the denitrification characteristics of the strain were further studied. The strain was identified by 16S rDNA sequence analysis, the effects of temperature, salinity, pH, substrate concentration and C/N ratio on denitrification activity were studied. Introduction Denitrifying bacteria are a kind of bacteria that can cause denitrification. Biological denitrification is the use of denitrifying bacteria, nitrate nitrogen into gaseous products to remove, is considered to be the most economical and effective way of nitrogen removal [1]-[2]. The application of denitrifying bacteria and the removal of nitrate in water have become the research focus[3]-[5], many methods have been reported [6]-[8]. In recent years, some studies on salt tolerant and halophilic denitrifying bacteria have been reported abroad [9]-[10]. In this study, the sludge from the culture pond was taken as the main separation source, and the enrichment, separation and purification were carried out.To obtain a highly efficient nitrate removal ability of denitrifying bacteria, named strain DN20. The aim of this study is to provide effective bacteria source for practical application of nitrate-N treatment in aquaculture water. -
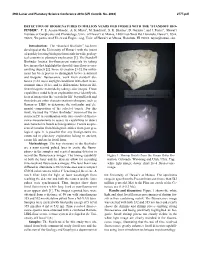
Detection of Biosignatures in Million Years Old Fossils with the “Standoff Bio- Finder”
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 2777.pdf DETECTION OF BIOSIGNATURES IN MILLION YEARS OLD FOSSILS WITH THE “STANDOFF BIO- FINDER”. T. E. Acosta-Maeda1, A. K. Misra1, M. Sandford1, S. K. Sharma1, D. Garmire2, and J. Porter1, 1Hawaiʻi Institute of Geophysics and Planetology, Univ. of Hawaiʻi at Mānoa, 1680 East-West Rd, Honolulu, Hawaiʻi, USA, 96822; 2Department of Electrical Engineering, Univ. of Hawaiʻi at Mānoa, Honolulu, HI 96822. [email protected]. Introduction: The “Standard Biofinder” has been developed at the University of Hawaiʻi with the intent of quickly locating biological materials in wide geolog- ical contexts in planetary exploration [1]. The Standoff Biofinder locates bio-fluorescent materials by taking live images that highlight the short lifetime fluorescence emitting objects [2]. Since its creation [3-5], the instru- ment has been proven to distinguish between mineral and biogenic fluorescence, work from standoff dis- tances (1-10 m) in daylight conditions with short meas- urement times (0.1s), and to differentiate between dif- ferent biogenic materials by taking color images. These capabilities could help an exploration rover identify ob- jects of interest for the ‘search for life’ beyond Earth and then dedicate other characterization techniques, such as Raman or LIBS, to determine the molecular and ele- mental composition of the selected targets. For this work, we used the “Color Biofinder” version of the in- strument [5] in combination with time-resolved fluores- cence measurements to assess its capabilities to detect and characterize fossils as biosignatures. Fossils are pre- served remains from biological entities from past geo- logical ages.