Phylogenetic Analysis and Review of Panacea and Batesia Butterflies N( Ymphalidae) Ryan I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inside: Idaea Asceta (Prout) (Geometridae), New to the U.S
________________________________________________________________________________________ Volume 53, Number 3 Fall 2011 www.lepsoc.org ________________________________________________________________________________________ Inside: Idaea asceta (Prout) (Geometridae), new to the U.S. Lepidoptera conserva- tion under a changing climate Karl Jordan Award win- ner: Don Lafontaine Life history of Leona’s Little Blue Tiputini Biological Sta- tion, Ecuador Late Season trip to the Richardson Mountains Membership Updates, The Mailbag, Marketplace... ... and more! ________________________________________________________________________________________ ________________________________________________________ Contents ________________________________________________________www.lepsoc.org A Late Season Trip to the Richardson Mountains ____________________________________ Michael Leski. ............................................................................................ 75 Volume 53, Number 3 Report on the Southern Lepidoptersists’ Society and Association Fall 2011 for Tropical Lepidoptera Meeting 2011 The Lepidopterists’ Society is a non-profit ed- Jacqueline Y. Miller. ............................................................................. 78 ucational and scientific organization. The ob- Idaea asceta (Prout) (Geometridae: Sterrhinae) from Texas, new ject of the Society, which was formed in May to the North American fauna 1947 and formally constituted in December Charles V. Covell. ................................................................................... -

Final Report for the University of Nottingham / Operation Wallacea Forest Projects, Honduras 2004
FINAL REPORT for the University of Nottingham / Operation Wallacea forest projects, Honduras 2004 TABLE OF CONTENTS FINAL REPORT FOR THE UNIVERSITY OF NOTTINGHAM / OPERATION WALLACEA FOREST PROJECTS, HONDURAS 2004 .....................................................................................................................................................1 INTRODUCTION AND OVERVIEW ..............................................................................................................................3 List of the projects undertaken in 2004, with scientists’ names .........................................................................4 Forest structure and composition ..................................................................................................................................... 4 Bat diversity and abundance ............................................................................................................................................ 4 Bird diversity, abundance and ecology ............................................................................................................................ 4 Herpetofaunal diversity, abundance and ecology............................................................................................................. 4 Invertebrate diversity, abundance and ecology ................................................................................................................ 4 Primate behaviour........................................................................................................................................................... -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

First Evidence for an Amazonian Insect Migration in the Butterfly Panacea Prola
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.01.277665; this version posted September 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 2 3 4 5 6 First evidence for an Amazonian insect migration in the butterfly Panacea prola 7 (Lepidoptera: Nymphalidae) 8 9 GEOFFREY GALLICE1,2,*, RICCARDO MATTEA1, & ALLISON STOISER1 10 1Alliance for a Sustainable Amazon, 7224 Boscastle Ln., Hanover, MD 21076, USA 11 2McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, 12 University of Florida, Gainesville, FL 32611, USA 13 *Correspondence [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.01.277665; this version posted September 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 14 ABSTRACT 15 16 Insect migrations rival those of vertebrates in terms of numbers of migrating individuals and 17 even biomass, although instances of the former are comparatively poorly documented. This is 18 especially true in the world’s tropics, which harbor the vast majority of Earth’s insect species. 19 Understanding these mass movements is of critical and increasing importance as global climate 20 and land use change accelerate and interact to alter the environmental cues that underlie 21 migration, particularly in the tropics. -

An Annotated Checklist of Ecuadorian Pieridae (Lepidoptera, Pieridae) 545-580 ©Ges
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Atalanta Jahr/Year: 1996 Band/Volume: 27 Autor(en)/Author(s): Racheli Tommaso Artikel/Article: An annotated checklist of Ecuadorian Pieridae (Lepidoptera, Pieridae) 545-580 ©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (December 1996) 27(3/4): 545-580, Wurzburg, ISSN 0171-0079 An annotated checklist of Ecuadorian Pieridae (Lepidoptera, Pieridae) by To m m a s o R a c h e li received 21.111.1996 Abstract: An account of 134 Pierid taxa occurring in Ecuador is presented. Data are from 12 years field experience in the country and from Museums specimens. Some new species records are added to Ecuadorian fauna and it is presumed that at least a 10% more of new records will be obtained in the near future. Ecuadorian Pieridae, although in the past many taxa were described from this country, are far from being thoroughly known. One of the most prolific author was Hewitson (1852-1877; 1869-1870; 1870; 1877) who described many species from the collections made by Buckley and Simons . Some of the "Ecuador” citations by Hewitson are pointed out more precisely by the same author (Hewit son , 1870) in his index to the list of species collected by Buckley in remote areas uneasily reached even to-day (V ane -Wright, 1991). An important contribution on Lepidoptera of Ecuador is given by Dognin (1887-1896) who described and listed many new species collected by Gaujon in the Loja area, where typical amazonian and páramo species are included. -

Classical Biocontrol: Panacea Or Pandora's Box1
Vol. 24, Nos. 2 & 3, October 15,1983 239 Classical Biocontrol: Panacea or Pandora's Box1 FRANCIS G. HOWARTH2 Like Gaul, biological control (or biocontrol) can be divided into 3 parts: cultural practices which enhance beneficial species already present; mass rearing of selected beneficial species for release against a pest; and, what has been termed classical biocontrol, the discovery, importation and release of a foreign species with the expectation that it will control a pest population. My comments will primarily concern classical biocontrol and stem from my experience as curator of the Hawaiian insect collection at the Bishop Museum, my research on native Hawaiian ecosystems, and my concern for conservation of native and endangered species. I thank W.C. Gagne for many helpful discussions during the preparation of this paper. Classical biological control blossomed in Hawaii in the late 19th and early 20th century, when a great many animals were indiscriminantly introduced, and the method has been used with some impressive economic successes both in Hawaii and elsewhere ever since. Enthusiasm for the method has resurged during the last 2 decades, after the fall from favor of chemical pest control. I share some of the enthusiasm towards the prospects of biocontrol, and it is not my position here to belittle the impressive successes being made in the control of economic pests. However, I am alarmed by several recent treatments and reviews on classical biocontrol that have touted the method as being without environmental risks. For example, DeBach (1974) stated that ". .. no adverse effects on the ecosystem occur from biological control." Doutt (1972) called the method "environmentally safe". -
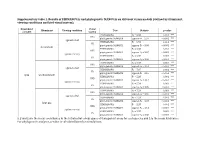
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

The Biology of Batesia Hypochlora in an Ecuadorian Rainforest (Lepidoptera: Nymphalidae) P
Vol. 10 No. 2 1999 et al: Biology of Batesia hypochlora 43 TROPICAL LEPIDOPTERA, 10(2): 43-46 (2000) THE BIOLOGY OF BATESIA HYPOCHLORA IN AN ECUADORIAN RAINFOREST (LEPIDOPTERA: NYMPHALIDAE) P. J. DEVRIES, C. M. PENZ, AND T. R. WALLA Dept. of Biology, University of Oregon, Eugene, Oregon 90430, USA ABSTRACT- Notes on the adult population biology and early stages of Batesia hypochlora are presented for the first time. The general natural history, population abundance, warning coloration, behavior, geographical distribution and hostplant use of Batesia hypochlora is discussed. Notes on the taxonomic relationship of Batesia and Panacea are also provided. KEYWORDS: Amazon, Batesia, Bolivia, Brazil, Caligo, Callicore, Catonephele, Colombia, ecology, Ecuador, Epiphile, Euphorbiaceae, fruit-feeding nymphalids, hostplants, immature stages, larvae, life history. Neotropical, Nessaea, Panacea, Peru, population biology, pupae, seasonally. South America, warning coloration. The Neotropical nymphalid butterfly genus Batesia Felder & modern human civilization. Thus our observations should be broadly Felder embraces the single, uniquely colored species Batesia relevant to populations of B. hypochlora occurring within intact hypochlora (Felder & Felder, 1862). This species inhabits lowland forest. rainforests from central Colombia to eastern Ecuador through Adults southeast Peru and western Brazil, and likely into northeast Bolivia; We observed adults feeding only on juices of rotting fruits, in effect, an upper Amazonian distribution. Despite the fact that B. wounds in trees, and very occasionally on fresh mammal dung or hypochlora is easily identified in the field (see illustrations in Lewis, rotting mammal carcasses. During a five year period (DeVries et ai, 1973; D'Abrera, 1987) and has long been popular with collectors, 1999, and unpubl.), we trapped 104 individuals of B. -

Volatile Cues Influence Host-Choice in Arthropod Pests
animals Review Volatile Cues Influence Host-Choice in Arthropod Pests Jacqueline Poldy Commonwealth Scientific and Industrial Research Organisation, Health & Biosecurity, Black Mountain Laboratory, Canberra, ACT 2601, Australia; [email protected]; Tel.: +61-2-6218-3599 Received: 1 October 2020; Accepted: 22 October 2020; Published: 28 October 2020 Simple Summary: Many significant human and animal diseases are spread by blood feeding insects and other arthropod vectors. Arthropod pests and disease vectors rely heavily on chemical cues to identify and locate important resources such as their preferred animal hosts. Although there are abundant studies on the means by which biting insects—especially mosquitoes—are attracted to humans, this focus overlooks the veterinary and medical importance of other host–pest relationships and the chemical signals that underpin them. This review documents the published data on airborne (volatile) chemicals emitted from non-human animals, highlighting the subset of these emissions that play a role in guiding host choice by arthropod pests. The paper exposes some of the complexities associated with existing methods for collecting relevant chemical features from animal subjects, cautions against extrapolating the ecological significance of volatile emissions, and highlights opportunities to explore research gaps. Although the literature is less comprehensive than human studies, understanding the chemical drivers behind host selection creates opportunities to interrupt pest attack and disease transmission, enabling more efficient pest management. Abstract: Many arthropod pests of humans and other animals select their preferred hosts by recognising volatile odour compounds contained in the hosts’ ‘volatilome’. Although there is prolific literature on chemical emissions from humans, published data on volatiles and vector attraction in other species are more sporadic. -

Molecular Phylogeny and Systematics of the Pieridae (Lepidoptera: Papilionoidea): Higher Classification and Biogeography
Blackwell Publishing LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2006? 2006 147? 239275 Original Article PHYLOGENY AND SYSTEMATICS OF THE PIERIDAEM. F. BRABY ET AL. Zoological Journal of the Linnean Society, 2006, 147, 239–275. With 8 figures Molecular phylogeny and systematics of the Pieridae (Lepidoptera: Papilionoidea): higher classification and Downloaded from https://academic.oup.com/zoolinnean/article-abstract/147/2/239/2631026 by Harvard Library user on 21 November 2018 biogeography MICHAEL F. BRABY1,2*, ROGER VILA1 and NAOMI E. PIERCE1 1Museum of Comparative Zoology, Harvard University, 26 Oxford St, Cambridge, MA 02138, USA 2School of Botany and Zoology, The Australian National University, Canberra, ACT 0200, Australia Received May 2004; accepted for publication October 2005 The systematic relationships of the butterfly family Pieridae are poorly understood. Much of our current under- standing is based primarily on detailed morphological observations made 50–70 years ago. However, the family and its putative four subfamilies and two tribes, have rarely been subjected to rigorous phylogenetic analysis. Here we present results based on an analysis of molecular characters used to reconstruct the phylogeny of the Pieridae in order to infer higher-level classification above the generic level and patterns of historical biogeography. Our sample contained 90 taxa representing 74 genera and six subgenera, or 89% of all genera recognized in the family. Three complementary approaches were -

Do Butterflies Use “Hearing Aids”? Investigating the Structure and Function of Inflated Wing Veins in Nymphalidae
Do butterflies use “hearing aids”? Investigating the structure and function of inflated wing veins in Nymphalidae by Penghui (Carrie) Sun A thesis submitted to the Faculty of Graduate and Postdoctoral Affairs in partial fulfillment of the requirements for the degree of Master of Biology in Biology Carleton University Ottawa, Ontario © 2018 Penghui (Carrie) Sun Abstract Many butterfly species within the subfamily Satyrinae (Nymphalidae) have been informally reported to possess a conspicuous “inflated” or “swollen” subcostal vein on each forewing. However, the function and taxonomic diversity of these structures is unknown. This thesis comprises both experimental and comparative approaches to test hypotheses on the function and evolution of these inflated veins. A laser vibrometry study showed that ears in the common wood nymph, Cercyonis pegala, are tuned to sounds between 1-5 kHz and the inflated subcostal vein enhances sensitivity to these sounds. A comparative study showed that all species with inflated veins possess ears, but not all species with ears possess inflated veins. Further, inflated veins were better developed in smaller butterflies. This thesis provides the first evidence for the function of inflated wing veins in butterflies and supports the hypothesis that they function as aids to low frequency hearing. ii Acknowledgements I thank my supervisor Dr. Jayne Yack for the continued guidance and support, throughout my academic program and in beginning my career, as well as an inspired and newfound appreciation I never knew I could have for insects. I thank my committee members Dr. Jeff Dawson and Dr. Charles-Antoine Darveau for their guidance, advice, and support. I thank Dr.