(12) Patent Application Publication (10) Pub. No.: US 2013/0216506 A1 DISCHER Et Al
Total Page:16
File Type:pdf, Size:1020Kb
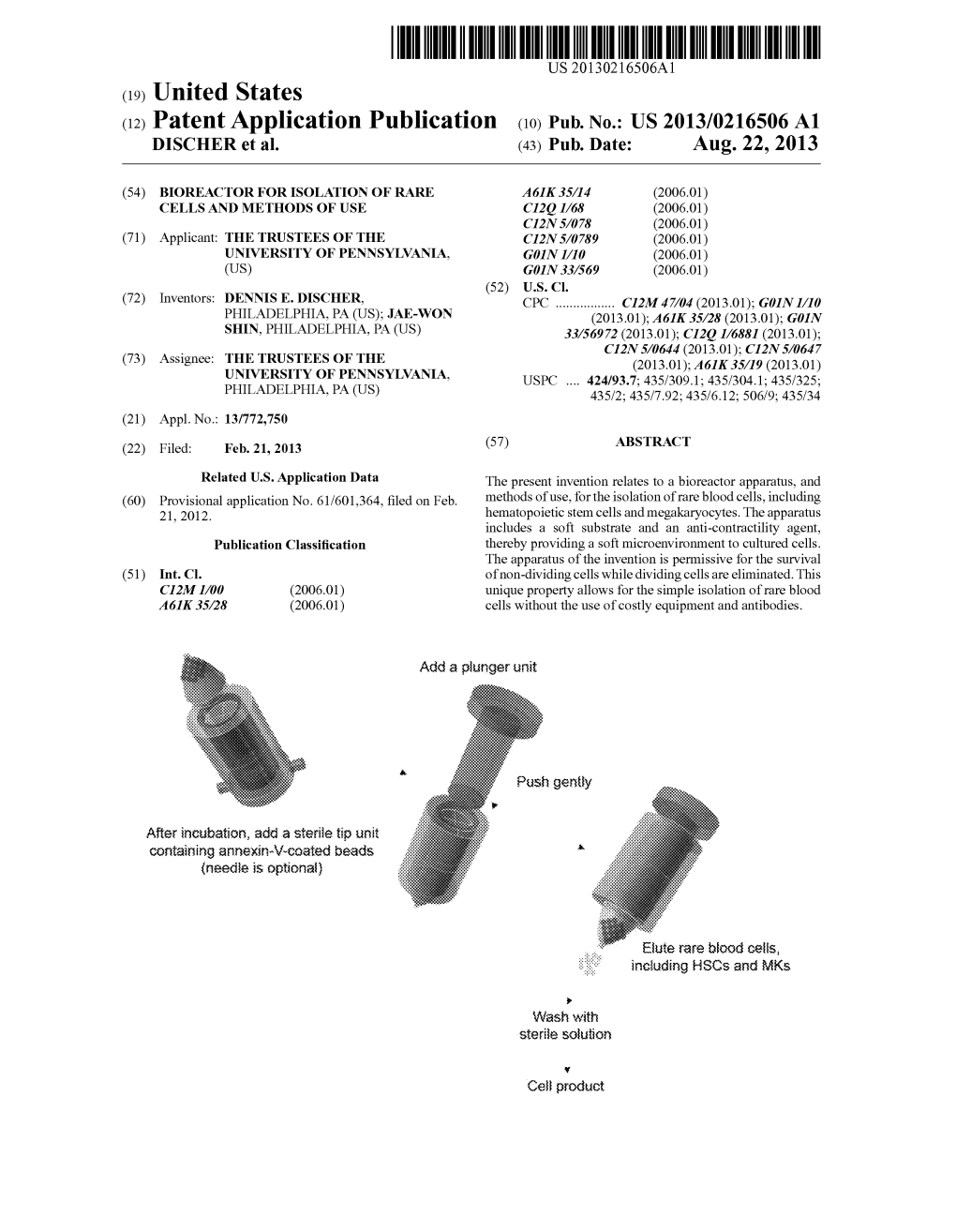
Load more
Recommended publications
-

A61K9/00 (2006.01) G01N 2 7/447 (2006.01) Chusetts 02139 (US)
( (51) International Patent Classification: (74) Agent: SCARR, Rebecca B. et al.; McNeill Baur PLLC, C07K 16/28 (2006.01) A61K 35/00 (2006.01) 125 Cambridge Park Drive, Suite 301, Cambridge, Massa¬ A61K9/00 (2006.01) G01N 2 7/447 (2006.01) chusetts 02139 (US). A61K9/19 (2006.01) C07K 19/00 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection av ailable) . AE, AG, AL, AM, PCT/US2020/036035 AO, AT, AU, AZ , BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (22) International Filing Date: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, 04 June 2020 (04.06.2020) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, (25) Filing Language: English KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (26) Publication Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (30) Priority Data: SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, TR, 62/857,364 05 June 2019 (05.06.2019) US TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. 62/906,862 27 September 2019 (27.09.2019) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: SEATTLE GENETICS, INC. [US/US]; kind of regional protection available) . ARIPO (BW, GH, 21823 30th Drive SE, Bothell, Washington 98021 (US). -

In Vivo Imaging of Tgfβ Signalling Components Using Positron
REVIEWS Drug Discovery Today Volume 24, Number 12 December 2019 Reviews KEYNOTE REVIEW In vivo imaging of TGFb signalling components using positron emission tomography 1 1 2 Lonneke Rotteveel Lonneke Rotteveel , Alex J. Poot , Harm Jan Bogaard , received her MSc in drug 3 1 discovery and safety at the Peter ten Dijke , Adriaan A. Lammertsma and VU University in 1 Amsterdam. She is Albert D. Windhorst currently finishing her PhD at the VU University 1 Department of Radiology and Nuclear Medicine, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands Medical Center (VUmc) 2 under the supervision of A. Pulmonary Medicine, Institute for Cardiovascular Research, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands D. Windhorst and Adriaan A. Lammertsma. Her 3 research interest is on the development of positron Department of Cell and Chemical Biology, Oncode Institute, Leiden University Medical Center, Leiden, The emission tomography (PET) tracers that target Netherlands selectively the activin receptor-like kinase 5 in vitro and in vivo. Alex J. Poot obtained his The transforming growth factor b (TGFb) family of cytokines achieves PhD in medicinal chemistry homeostasis through a careful balance and crosstalk with complex from Utrecht University. As postdoctoral researcher at signalling pathways. Inappropriate activation or inhibition of this pathway the VUmc, Amsterdam, he and mutations in its components are related to diseases such as cancer, developed radiolabelled anticancer drugs for PET vascular diseases, and developmental disorders. Quantitative imaging of imaging. In 2014, he accepted a research expression levels of key regulators within this pathway using positron fellowship from Memorial Sloan Kettering Cancer 13 emission tomography (PET) can provide insights into the role of this Center, New York to develop C-labelled probes for tumour metabolism imaging with magnetic resonance in vivo pathway , providing information on underlying pathophysiological imaging (MRI). -

How Relevant Are Bone Marrow-Derived Mast Cells (Bmmcs) As Models for Tissue Mast Cells? a Comparative Transcriptome Analysis of Bmmcs and Peritoneal Mast Cells
cells Article How Relevant Are Bone Marrow-Derived Mast Cells (BMMCs) as Models for Tissue Mast Cells? A Comparative Transcriptome Analysis of BMMCs and Peritoneal Mast Cells 1, 2, 1 1 2,3 Srinivas Akula y , Aida Paivandy y, Zhirong Fu , Michael Thorpe , Gunnar Pejler and Lars Hellman 1,* 1 Department of Cell and Molecular Biology, Uppsala University, The Biomedical Center, Box 596, SE-751 24 Uppsala, Sweden; [email protected] (S.A.); [email protected] (Z.F.); [email protected] (M.T.) 2 Department of Medical Biochemistry and Microbiology, Uppsala University, The Biomedical Center, Box 589, SE-751 23 Uppsala, Sweden; [email protected] (A.P.); [email protected] (G.P.) 3 Department of Anatomy, Physiology and Biochemistry, Swedish University of Agricultural Sciences, Box 7011, SE-75007 Uppsala, Sweden * Correspondence: [email protected]; Tel.: +46-(0)18-471-4532; Fax: +46-(0)18-471-4862 These authors contributed equally to this work. y Received: 29 July 2020; Accepted: 16 September 2020; Published: 17 September 2020 Abstract: Bone marrow-derived mast cells (BMMCs) are often used as a model system for studies of the role of MCs in health and disease. These cells are relatively easy to obtain from total bone marrow cells by culturing under the influence of IL-3 or stem cell factor (SCF). After 3 to 4 weeks in culture, a nearly homogenous cell population of toluidine blue-positive cells are often obtained. However, the question is how relevant equivalents these cells are to normal tissue MCs. By comparing the total transcriptome of purified peritoneal MCs with BMMCs, here we obtained a comparative view of these cells. -

A Cytokine Protein-Protein Interaction Network for Identifying Key Molecules in Rheumatoid Arthritis
RESEARCH ARTICLE A cytokine protein-protein interaction network for identifying key molecules in rheumatoid arthritis Venugopal Panga1,2, Srivatsan Raghunathan1* 1 Institute of Bioinformatics and Applied Biotechnology (IBAB), Biotech Park, Electronics City Phase I, Bengaluru, Karnataka, India, 2 Manipal Academy of Higher Education, Manipal, Karnataka, India * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Rheumatoid arthritis (RA) is a chronic inflammatory disease of the synovial joints. Though the current RA therapeutics such as disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs) and biologics can halt the progression of the disease, none of these would either dramatically reduce or cure RA. So, the identification of OPEN ACCESS potential therapeutic targets and new therapies for RA are active areas of research. Several Citation: Panga V, Raghunathan S (2018) A studies have discovered the involvement of cytokines in the pathogenesis of this disease. cytokine protein-protein interaction network for identifying key molecules in rheumatoid arthritis. These cytokines induce signal transduction pathways in RA synovial fibroblasts (RASF). PLoS ONE 13(6): e0199530. https://doi.org/ These pathways share many signal transducers and their interacting proteins, resulting in 10.1371/journal.pone.0199530 the formation of a signaling network. In order to understand the involvement of this network Editor: Hua Zhou, Macau University of Science and in RA pathogenesis, it is essential to identify the key transducers and their interacting pro- Technology, MACAO teins that are part of this network. In this study, based on a detailed literature survey, we Received: August 21, 2017 have identified a list of 12 cytokines that induce signal transduction pathways in RASF. -

Mapping Macrophage Polarization Over the Myocardial Infarction Time Continuum
Edinburgh Research Explorer Mapping macrophage polarization over the myocardial infarction time continuum Citation for published version: Mouton, AJ, DeLeon-Pennell, KY, Rivera Gonzalez, OJ, Flynn, ER, Freeman, TC, Saucerman, JJ, Garrett, MR, Ma, Y, Harmancey, R & Lindsey, ML 2018, 'Mapping macrophage polarization over the myocardial infarction time continuum', Basic research in cardiology, vol. 113, no. 4, pp. 26. https://doi.org/10.1007/s00395-018-0686-x Digital Object Identifier (DOI): 10.1007/s00395-018-0686-x Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Basic research in cardiology Publisher Rights Statement: This article is distributed under the terms of the Creative Commons Attribution, which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 07. -

Activemax® Recombinant Human TGF-Beta 1 / TGFB1 Catalog # AMS.TG1-H4212 for Research and Further Cell Culture Manufacturing Use
ActiveMax® Recombinant Human TGF-Beta 1 / TGFB1 Catalog # AMS.TG1-H4212 For Research and Further Cell Culture Manufacturing Use Description Source ActiveMax® Recombinant Human TGF-Beta 1 / TGFB1 (ActiveMax® Human TGF-Beta 1) Ala 279 - Ser 390 (Accession # NP_000651.3) was produced in human 293 cells (HEK293) Predicted N-terminus Ala 279 Molecular Characterization Endotoxin Less than 1.0 EU per μg of the ActiveMax® Human TGF-Beta 1 by the LAL method. Purity >95% as determined by SDS-PAGE of reduced (+) and non-reduced (-) rhTGFB1. Bioactivity The bio-activity was determined by its ability to inhibit IL-4 induced HT-2 cell proliferation. The ED50<0.05 ng/mL, corresponding to a specific activity of >2X107 Unit/mg Formulation and Storage Formulation Lyophilized from 0.22 μm filtered solution in TFA and acetonitrile. Normally Mannitol or Trehalose are added as protectants before lyophilization. Contact us for customized product form or formulation. Reconstitution See Certificate of Analysis for reconstitution instructions and specific concentrations. Storage Lyophilized Protein should be stored at -20℃ or lower for long term storage. Upon reconstitution, working aliquots should be stored at -20℃ or -70℃. Avoid repeated freeze-thaw cycles. No activity loss was observed after storage at: ● 4-8℃ for 12 months in lyophilized state; ● -70℃ for 3 months under sterile conditions after reconstitution. Background Background Transforming growth factor beta 1 ( TGFB1) is also known as TGF-β1, CED, DPD1, TGFB. is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation and apoptosis. -

Beclin 1 Regulates Neuronal Transforming Growth Factor-Β Signaling by Mediating Recycling of the Type I Receptor ALK5 Caitlin E
O’Brien et al. Molecular Neurodegeneration (2015) 10:69 DOI 10.1186/s13024-015-0065-0 RESEARCH ARTICLE Open Access Beclin 1 regulates neuronal transforming growth factor-β signaling by mediating recycling of the type I receptor ALK5 Caitlin E. O’Brien1,2,3, Liana Bonanno2,3,4, Hui Zhang2,3 and Tony Wyss-Coray2,3* Abstract Background: Beclin 1 is a key regulator of multiple trafficking pathways, including autophagy and receptor recycling in yeast and microglia. Decreased beclin 1 levels in the CNS result in neurodegeneration, an effect attributed to impaired autophagy. However, neurons also rely heavily on trophic factors, and signaling through these pathways requires the proper trafficking of trophic factor receptors. Results: We discovered that beclin 1 regulates signaling through the neuroprotective TGF-β pathway. Beclin 1 is required for recycling of the type I TGF-β receptor ALK5. We show that beclin 1 recruits the retromer to ALK5 and facilitates its localization to Rab11+ endosomes. Decreased levels of beclin 1, or its binding partners VPS34 and UVRAG, impair TGF-β signaling. Conclusions: These findings identify beclin 1 as a positive regulator of a trophic signaling pathway via receptor recycling, and suggest that neuronal death induced by decreased beclin 1 levels may also be due to impaired trophic factor signaling. Keywords: Beclin 1, VPS34, Retromer, TGF-β, ALK5, Protein sorting, Receptor recycling, Neurodegeneration Background Beclin 1 is highly expressed in the nervous system and Beclin 1 is a component of the type III is essential for neuronal survival. While beclin 1 knock- phosphatidylinositol-3-kinase (PI3K) complex. -

14Th International Conference on Behçet's Disease
14th International Conference on Behçet’s Disease London, United Kingdom, 8-10 July 2010 Executive Committee – International Society for Behçet’s Disease President Sungnack Lee (Korea) - Dermatology Past-President Hasan Yazici (Turkey) - Rheumatology Vice-President Kenneth Calamia (USA) - Rheumatology Secretary Dongsik Bang (Korea) - Dermatology Treasurer Samir Assaad Khalil (Egypt) - Internal Medicine President of 14th International Congress Dorian Haskard (UK) - Rheumatology President Past International Congress Michael Schirmer (Austria) - Rheumatology Member – Dermatology Eun-So Lee (Korea) - Dermatology Member – Internal Medicine Petros Sfikakis (Greece) - Internal Medicine Member – Scientific Affairs Haner Direskeneli (Turkey) - Rheumatology Member – Publication Affairs Graham Wallace (UK) - Immunology Hon. Life Presidents – International Society for Behçet’s Disease Colin G. Barnes (UK) - Rheumatology Nihat Dilsen (Turkey) - Rheumatology George Ehrlich (USA) - Rheumatology Thomas Lehner (UK) - Immunology Desmond O’Duffy (USA) - Rheumatology Local Organising Committee Honorary President Dorian Haskard (UK) - Rheumatology International Affairs Secretary Colin G. Barnes (UK) - Rheumatology Honorary General Secretary Graham Wallace (UK) - Immunology Farida Fortune (UK) - Oral Medicine Robert Moots (UK) - Rheumatology Miles Stanford (UK) - Ophthalmology Representatives – Behçet’s Syndrome Society Jan Mather (UK) - BSS Chris Phillips (UK) - BSS Abstracts page Oral Presentation index S-107 Oral Presentations S-108 Poster Presentations index -

Signalwege – Fakultatives Material – -Fakultativ- 1 Die Folgenden
Signalwege – Fakultatives Material – Die folgenden Texte stammen aus Wikipedia unmodifiziert, deutsch-englisch gemischt. Die Schriftgrösse könnt Ihr Euch nach belieben einteilen. Die vorliegende Schriftgrösse 8 spart Papier und ist sehr übersichtlich, was meiner Meinung nach das Lesetempo und Wiederholungen maximal beschleunigt. G-Protein-gekoppelter Rezeptor aus Wikipedia, der freien Enzyklopädie Wechseln zu: Navigation , Suche Der Begriff G-Protein-gekoppelter Rezeptor (kurz GPCR , für englisch G protein-coupled receptor ) wird in der Biologie für Rezeptoren in der Zellmembran verwendet, die Signale über GTP -bindende Proteine (kurz G-Proteine ) in das Zellinnere weiterleiten (Signaltransduktion ). Diese stellen die größte und vielseitigste Gruppe von Membranrezeptoren dar. In der Neurobiologie wird für G-Protein-gekoppelte Rezeptoren häufig der Begriff metabotrope Rezeptoren verwendet, um sie von einem anderen Rezeptortyp, den ligandengesteuerten Ionenkanälen (Ionotroper Rezeptor), zu unterscheiden. G-Protein-gekoppelte Rezeptoren sind für die Verarbeitung von Licht -, Geruchs - und einer Vielzahl von Geschmacksreizen verantwortlich. Sie spielen eine entscheidende Rolle bei Entzündungsprozessen , der gezielten Zellbewegung (Chemotaxis ), dem Transport von Stoffen durch die Zellmembran (Endozytose und Exozytose ) sowie beim Zellwachstum und bei der Zelldifferenzierung . Sie sind darüber hinaus als Zielstrukturen für die Wirkung von Hormonen , wie Adrenalin oder Glucagon , und Neurotransmittern , wie Serotonin und Acetylcholin , verantwortlich. Auch einige Viren nutzen G-Protein-gekoppelte Rezeptoren als Bindungsstellen für den Eintritt in die Zelle (beispielsweise HIV ). Definition Als G-Protein-gekoppelte Rezeptoren im engeren Sinn werden alle heptahelikalen, das heißt mit 7 Helices in der Zellmembran verankerten Rezeptoren bezeichnet, die zur Bindung und Aktivierung von G-Proteinen befähigt sind. Zusätzlich zur Bindung von G-Proteinen sind viele dieser Rezeptoren in der Lage, auch mit anderen signalweiterleitenden Proteinen zu interagieren. -

The Pennsylvania State University the Graduate School the Huck Institutes for Life Sciences MODULATION of NUCLEAR FACTOR KAPPA B
The Pennsylvania State University The Graduate School The Huck Institutes for Life Sciences MODULATION OF NUCLEAR FACTOR KAPPA B (NFκB) TRANSACTIVATION BY TRANSFORMING GROWTH FACTOR β-1 (TGFβ-1) IN KERATINOCYTES: IMPLICATIONS FOR RESPONSIVENESS TO ULTRAVIOLET RADIATION (UVB) A Dissertation in Integrative Biosciences by Kelly A. Hogan ©2011 Kelly A. Hogan Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2011 The dissertation of Kelly A. Hogan was reviewed and approved* by the following: Adam B. Glick Associate Professor of Veterinary and Biomedical Sciences Dissertation Advisor Chair of Committee John Vanden Heuvel Professor of Veterinary and Biomedical Sciences Andrea Mastro Professor of Microbiology and Cell Biology K. Sandeep Prabhu Associate Professor of Immunology and Molecular Toxicology Avery August Distinguished Adjunct Professor of Immunology Peter Hudson Willaman Professor of Biology Director, Huck Institutes of the Life Sciences *Signatures are on file in the Graduate School ii Abstract Molecular crosstalk leading to the integration of signal transduction pathways—and the formation of a signaling network—is particularly important for maintaining cellular homeostasis. Stimuli received at the cell surface and transduced to the nucleus can expect to be modified by any number of inputs in a highly context-dependent and cell-specific manner. Nuclear factor kappa B (NFκB) and transforming growth factor β-1 (TGFβ-1) are not only critical factors mediating inflammation, but they also play a substantial role in cancer progression. Therefore, understanding the intersection of these factors may shed light on inflammatory diseases and progression of cancer in skin. However, little is known about how TGFβ-1 and NFκB interact in keratinocytes, which rely heavily on both factors to maintain homeostasis. -

Inflammatory, Regulatory, and Autophagy Co-Expression Modules
Durocher et al. Journal of Neuroinflammation (2019) 16:56 https://doi.org/10.1186/s12974-019-1433-4 RESEARCH Open Access Inflammatory, regulatory, and autophagy co-expression modules and hub genes underlie the peripheral immune response to human intracerebral hemorrhage Marc Durocher1, Bradley P. Ander1, Glen Jickling1, Farah Hamade1, Heather Hull1, Bodie Knepp1, Da Zhi Liu1, Xinhua Zhan1, Anh Tran1, Xiyuan Cheng1, Kwan Ng1, Alan Yee1, Frank R. Sharp1 and Boryana Stamova1,2* Abstract Background: Intracerebral hemorrhage (ICH) has a high morbidity and mortality. The peripheral immune system and cross-talk between peripheral blood and brain have been implicated in the ICH immune response. Thus, we delineated the gene networks associated with human ICH in the peripheral blood transcriptome. We also compared the differentially expressed genes in blood following ICH to a prior human study of perihematomal brain tissue. Methods: We performed peripheral blood whole-transcriptome analysis of ICH and matched vascular risk factor control subjects (n = 66). Gene co-expression network analysis identified groups of co-expressed genes (modules) associated with ICH and their most interconnected genes (hubs). Mixed-effects regression identified differentially expressed genes in ICH compared to controls. Results: Of seven ICH-associated modules, six were enriched with cell-specific genes: one neutrophil module, one neutrophil plus monocyte module, one T cell module, one Natural Killer cell module, and two erythroblast modules. The neutrophil/monocyte modules were enriched in inflammatory/immune pathways; the T cell module in T cell receptor signaling genes; and the Natural Killer cell module in genes regulating alternative splicing, epigenetic, and post-translational modifications. -

The Role of Integrin Αvβ8 on Human Monocytes and Macrophages in Intestinal Immune Homeostasis
The role of integrin αvβ8 on human monocytes and macrophages in intestinal immune homeostasis A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy in the Faculty of Biology, Medicine and Health 2018 Elinor Elizabeth Shuttleworth School of Biological Sciences TABLE OF CONTENTS Chapter 1: Introduction .................................................................................. 15 1.1. Introduction ..................................................................................................... 15 1.2. Intestinal barrier function ............................................................................... 15 1.2.1. Intestinal epithelial layer ............................................................................. 17 1.2.2. PRR function in IECs .................................................................................. 19 1.2.3. Intestinal mucus layer ................................................................................. 20 1.3. Intestinal innate immunity .............................................................................. 20 1.3.1. Monocytes and Macrophages .................................................................... 21 1.3.2. Dendritic cells ............................................................................................. 35 1.4. Intestinal adaptive immunity .......................................................................... 41 1.4.1. Intestinal T cells .........................................................................................