This Version Replaces a Previous
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Laboratory Doctors
Laboratory & Diagnosis Official Journal of Iranian Association of Clinical Laboratory Doctors Editorial Manager: Dr. Mohammad Sahebalzamani, DCLS Editor in Chief: Dr. S. Mahdi Bolourchi, DCLS Editorial Board Members: Dr. Mohammad Reza Bakhtiari, DCLS, PhD Dr. Davood Behravan, DCLS Dr. S. Mahdi Bolourchi, DCLS Dr. Behzad Poopak, DCLS, PhD Dr. Majid Jalilzadeh Khoei, DCLS Dr. S. Mohammad Hasan Hashemimadani, DCLS Dr. Ali Sadeghitabar, DCLS Dr. Mohammad Sahebalzamani, DCLS Dr. Mohammad Javad Soltanpour, DCLS Executive Board Members: S. Farzaneh Bathaei Sara Tondro Abolfazl Yousefian Navid Ghahremani Tahereh Komasi Circulation: 3000 Copies Address: No.29, Ardeshir Alley, Hashtbehesht St., Golha Square, Fatemi Ave, Tehran 1414734711 – Iran. Telefax: (+98 21) 88970700 Laboratory & Diagnosis Vol.3, No14, Suplememt Issue Massage of Congress Chairman After several months passed over the 4th international and 9th national congress on quality improvement in clinical laboratories, also gaining valuable experiences and reviewing over benefits and disadvantaging points, now there is a new chance to pro- vide The 5th international & 10th national congress, and all these opportunities are available now because of GODs grace. Congress efforts are done to improve quality of laboratory services by providing appropriate environment for intellectual agreement, information exchange, presenting the results of different researches and sharing updated scientific information of Iranian and abroad professors, elites, colleagues. Extending and optimizing laboratory services in different branches of clinical laboratory sciences as desired of society requirement are the main objectives of congress. We hope all those who are involved in various fields of laboratory sciences either in Iran or abroad consider to take part in this splendid scientifically stage and give us this chance to take advantage of their knowledge and experiences. -

In the Name of God HISTORY
In The Name of God HISTORY The Packman Company was established in February 1975. In that year it was also registered in Tehran›s Registration Department. Packman›s construction and services company was active in building construction and its services in the early years of its formation.In 1976 in cooperation with (Brown Boveri and Asseck companies) some power plant mega projects was set up by the compa- ny.The company started its official activity in the filed of construction of High-Pressure Vessels such as Hot-Water Boilers , Steam Boilers , Pool Coil Tanks Softeners and Heat Exchangers from 1984. Packman Company was one of the first companies which supplied its customers with hot- water boilers which had the quality and standard mark.Packman has been export- ing its products to countries such as Uzbekistan, United Arab Emirates and other countries in the region. It is one of the largest producers of hot-water and steam boilers in the Middle East. Packman Company has got s degree from the Budget and Planning Organization in construction and services in the membership of some important associations such as: 1. Construction Services Industry Association 2. Industry Association 3. Construction Companies› Syndicate 4.Technical Department of Tehran University›s Graduates Association 5. Mechanical Engineering Association 6. Engineering Standard Association Packman Product: Steam boiler ( Fire tube ) Hot water boiler ( Fire tube ) Combination boile Water pack boiler Steam boiler ( water tube ) Hot water boiler (water tube) Boiler accessories Pressure vessel Water treatment equipment SOME OF CERTIFICATION ARE Manufacturer of Boilers, Thermal Oil Heaters, Heat Exchangers, Pressure Vessels, Storage Tanks & Industrial Water Treatment Equipments ,.. -
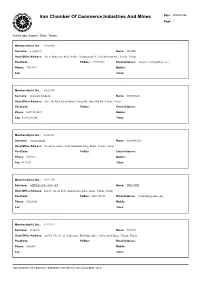
Iran Chamber of Commerce,Industries and Mines Date : 2008/01/26 Page: 1
Iran Chamber Of Commerce,Industries And Mines Date : 2008/01/26 Page: 1 Activity type: Exports , State : Tehran Membership Id. No.: 11020060 Surname: LAHOUTI Name: MEHDI Head Office Address: .No. 4, Badamchi Alley, Before Galoubandak, W. 15th Khordad Ave, Tehran, Tehran PostCode: PoBox: 1191755161 Email Address: [email protected] Phone: 55623672 Mobile: Fax: Telex: Membership Id. No.: 11020741 Surname: DASHTI DARIAN Name: MORTEZA Head Office Address: .No. 114, After Sepid Morgh, Vavan Rd., Qom Old Rd, Tehran, Tehran PostCode: PoBox: Email Address: Phone: 0229-2545671 Mobile: Fax: 0229-2546246 Telex: Membership Id. No.: 11021019 Surname: JOURABCHI Name: MAHMOUD Head Office Address: No. 64-65, Saray-e-Park, Kababiha Alley, Bazar, Tehran, Tehran PostCode: PoBox: Email Address: Phone: 5639291 Mobile: Fax: 5611821 Telex: Membership Id. No.: 11021259 Surname: MEHRDADI GARGARI Name: EBRAHIM Head Office Address: 2nd Fl., No. 62 & 63, Rohani Now Sarai, Bazar, Tehran, Tehran PostCode: PoBox: 14611/15768 Email Address: [email protected] Phone: 55633085 Mobile: Fax: Telex: Membership Id. No.: 11022224 Surname: ZARAY Name: JAVAD Head Office Address: .2nd Fl., No. 20 , 21, Park Sarai., Kababiha Alley., Abbas Abad Bazar, Tehran, Tehran PostCode: PoBox: Email Address: Phone: 5602486 Mobile: Fax: Telex: Iran Chamber Of Commerce,Industries And Mines Center (Computer Unit) Iran Chamber Of Commerce,Industries And Mines Date : 2008/01/26 Page: 2 Activity type: Exports , State : Tehran Membership Id. No.: 11023291 Surname: SABBER Name: AHMAD Head Office Address: No. 56 , Beside Saray-e-Khorram, Abbasabad Bazaar, Tehran, Tehran PostCode: PoBox: Email Address: Phone: 5631373 Mobile: Fax: Telex: Membership Id. No.: 11023731 Surname: HOSSEINJANI Name: EBRAHIM Head Office Address: .No. -
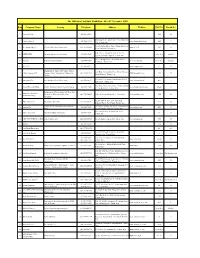
Row Company Name Activity Telephone Address Website Hall No Booth No
The 10th Auto Parts Int,l. Exhibition - 16 to 19 November 2015 Row Company Name Activity Telephone Address WebSite Hall No Booth No 1 Abarashi Group (021)36466786 31B 38 D46 Golgasht St., Golzar Ave, Parand Industrial 2 Abzar Andisheh (021)56419014 www.abzarandisheh.com 40B 7 City, Tehran- Iran No.120, Kalhor Blvd, Shahre Ghods, 20th km of 3 Ace Engineering Co Electrical Auto Part Manufacturer (021)46884888-9 www.ACE.IR 40B 16 Karaj Old Road, Tehran, Iran Unit 2, No. 4, Koopayeh Alley, Before the 4 ADIB IMENi Garment industry and advertising (021)55380846 Open Area South 31 Qazvin Sq, South Kargar St, Tehran, Iran No. 17, Dastgheib Ave, West Shahed Blvd., 5 Agradad Industrial Automatic Door (021)44588684 www.agradad.com Open Area South 31 Tehransar, Tehran, Iran 6 AL.TECH. (021)26760992 www.dinapart.com 6 38 Manufactur of Types of Steel Parts by hot Sarir Bldg., Peykanshahr Exit,15th km Tehran- 7 Alborz Forging IND forging method, Auto Gearbox, Suspension (021)44784191-5 www.forgealborz.com 40B 29 Karaj Highway, Tehran- Iran Chassis No. 18 & 19, Next to the Gas Station, West 15 8 Aluminium Faz Car Aluminium Parts (Die Casting) (021)55690137 www.aluminiumfaz.ir 40A 3 Khordad St., Tehran, Iran First Floor, No.7, Zahiroleslam Alley, Iranshahr 9 Alvand Electronic Dana Vehicle Tracking, kinds of electronic boards (021)88313640 www.alvandelectronic.com 20-22 16 St., Taleghani Ave., Tehran- Iran Production of different kind of oil filters, Fuel Aman Filter Industrial 10 filters & Air filters for light & heavy (021)77167003-5 Unit 6, 3rd Floor, Piroozi Ave, Tehran, Iran www.amanfilter.com 31B 28 Production Group automobile No.207, 208- F, Sarv 24 St, Nasirabad 11 Aman Ghate Kar Automobile spare parts (021)56390795 20-22 20 Industrial Town, Saveh Road, Tehran, Iran Manufacturing Auto suspension & steering 1st Eastern 20 Meter St., Tabriz Exhibition old 12 Amirnia Co. -
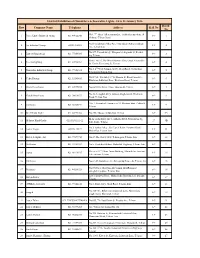
Row Company Name Telephone Address Hall No Booth No 23Rd Intl
23rd Intl Exhibition of Chandeliers & Decorative Lights - 10 to 13 January 2016 Booth Row Company Name Telephone Address Hall No No No9, 7th Aban Alley, zamyad st , irankhodro junction, 14 1 Parsa Luster Industrial Group 021 44922243 8-9 1 kh karaj- Tehran Road No.ll.Valadkhani Alley.After Marzdaran.Ashrafi.Isfahani 2 Far Industrial Group 02155470325 8-9 2 Ave.Tehran.Iran No.577, Pirhadi Alley , Hengam St, Alghadir St, Resalat 3 Lustert Shaghayegh 021 77806936 8-9 3 Sq, Tehran Unit8, No.25, The West Khosroo Alley, Ostad Nejat olahi 4 Paeezan lighting 021 88910762 8-9 4 St, Karim Khan zand St, Tehran No.1, 2nd West Sazman Ab St, Ab ali Road, T-Junction 5 Noorsazan Industrial Group 021 77353333 8-9 5 Tehranpars,Tehran, Iran NO.3714, Yas Alley, 1st St, Koosar St, Fan avaran Site, 6 Yekta Boronz 021 33283410 8-9 6 Khavaran Industrial Zone, Khavaran Road, Tehran 7 Kham Peres Luster 021 33979855 No.66,Marvi Bazar, Naser khosroo St, Tehran 8-9 7 No.16, Sedaghat Alley, Abbass Alaghemand , Khavaran 8 Choob Noor Parsa 021 36676833 8-9 8 Road, Tehran, Iran No. 1, Senoubar2,Emam reza 32, Khatoun abad, Pakdasht, 9 Parsbronz 021 36460194 8-9 9 Tehran 10 Steel Brons Haghi 021 66971206 No.858, Abo saeed Junction, Tehran 8-9 9A Mehr ara Industry Alley, Alghadir Blvd, Nabi akram Sq, 11 Mehrara Hand Grafts 02165296211-12 27 9B Safa dasht , Tehran No.1, Janbaz Alley , Gol Tapeh Kabir, Varamin Road, 12 Luster Negin 02136148479 8-9 11 Shahr Ray, Tehran, Iran 13 Kaveh Sculpture Art 021 77879750 No.47, The East 174 St ,Tehran pars, Tehran, Iran 8-9 12 14 Gift Fakor 021 33723594 No.5, Dead-End Ehdasi, Mahallati Highway, Tehran, Iran 8-9 13 The west 2 nd Floor, Amin Building, Manochehri Junction, 15 Espoo 021 66170195 8-9 14 Lale zar noo, Tehran 16 Pak Luster No.61-59,Abrishami Ave,Behesh Sq,Rajaee St, Tehran, Iran 8-9 15 No.69.Naser Ghadyani Alley Amir AbasRahimi st 17 Roshanaie 021 44218921 8-9 16 sadeghiyeh square. -

Company Name Address City Workphone Fax / Email Interests Aboutorabian Trading 33, Mobasher Shopping Center, Opp
Company Name Address City WorkPhone Fax / Email Interests Aboutorabian Trading 33, Mobasher Shopping Center, Opp. Tehran 009821-33122401, 33122401,33122819;iraj_ra Food, Rice Seied Esmaeel, Sirus St., 33122819 [email protected], Ahmad Vadoudi Mofid Bldg.33, Apt.4, 32nd St., Shahrara Tehran 825 3598 +98-21-825 3414 Agro Product - Rice Akam Tosee 60, 7th Fath st., 3rd Km. of Karaj old Tehran 009821-66806501 66806997; Food, rice Road, (fath Highway), [email protected] Alborz Trading Apt. 6, 2nd. Fl,. No. 14, Ashhari St., Tehran 009821-66732120 66734850;sahebmax@gmai food, rice, Import Under Hafez Bridge,Jomhouri St., l.com Ali Bashari Doost Qum 933 807 +98-251-933 834 Agro - Rice Ali Iranmanesh Kerman [email protected] Food, rice Amir Keshmiri [email protected] Agro, food, rice, Arian Top Noosh Apt. 1, No.13, 13th St., Bokharest St., Tehran 009821-88700180 88703687;[email protected] Food, rice m Arvin Commercial Group Apt. 6, 8th Fl, PAM Tower, Zafar St., Tehran 009821-8889852 88799616; [email protected] Agro, food, brown rice Africa Blvd., Ata Tea 8, Monfared Alley, First of Abdollahi St., Tehran 009821-55631137 55818859; info@ata- Import of Food, rice & Tea Next to Akabrabadi Hospital, Molavi tea.com Atlas Business Service 6, 3rd Fl.,Haj Mohammadiyan Tehran ( ) 875-2595 021-876 9059 Agro Product - Rice - Wheat Alley,Parsa St., Motahari Balkhi World Wide Trading P.O.Box: 51335-1678 Tabriz 0098411-5235777 5235777; Food , rice Bari Trading Unit 2, No. 20, Kamran Alley, Rahi Tehran 009821-888955715 [email protected], Agro, Food, rice Moayeri St., Fatemi Ave., [email protected] BATITCO 112, Corner Saied Alley, somayeh st., Tehran 009821-88301094-7, 88827923; Food, rice, fruit Mousavi St., Taleghani St., [email protected] Bazargani Sabet, Mr. -

Post-Revolutionary Iran's Foreign Policy Toward the United
POST-REVOLUTIONARY IRAN’S FOREIGN POLICY TOWARD THE UNITED STATES: A HISTORICAL SOCIOLOGICAL ANALYSIS OF STATE TRANSFORMATION AND FOREIGN POLICY A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF SOCIAL SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY GÜLRİZ ŞEN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR DOCTOR OF PHILOSOPHY IN THE DEPARTMENT OF INTERNATIONAL RELATIONS SEPTEMBER 2013 Approval of the Graduate School of Social Sciences ________________________ Prof. Dr. Meliha Altunışık Director I certify that this thesis satisfies all the requirements as a thesis for the degree of Doctor of Philosophy. ________________________ Prof. Dr. Hüseyin Bağcı Head of Department This is to certify that we have read this thesis and that in our opinion it is fully adequate, in scope and quality, as a thesis for the degree of Doctor of Philosophy. ________________________ Prof. Dr. Meliha Altunışık Supervisor Examining Committee Members Assoc. Prof. Dr. Faruk Yalvaç (METU, IR) _________________________ Prof. Dr. Meliha Altunışık (METU, IR) _________________________ Prof. Dr. Recep Boztemur (METU, HIST) _________________________ Prof. Dr. Elisabeth Özdalga (BİLKENT, POLS) _________________________ Assoc. Prof. Dr. Özlem Tür (METU, IR) _________________________ I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name: Gülriz Şen Signature: iii ABSTRACT POST-REVOLUTIONARY IRAN’S FOREIGN POLICY TOWARD THE UNITED STATES: A HISTORICAL SOCIOLOGICAL ANALYSIS OF STATE TRANSFORMATION AND FOREIGN POLICY Şen, Gülriz Ph.D., Department of International Relations Supervisor: Prof. -

Annual Report 2010/11 Annual Report 2010/11
ANNUAL REPORT 2010/11 2010/11 REPORT ANNUAL The intelligent Bank [email protected] www.sb24.com 1 ANNUAL REPORT 2010/11 CONTENTS OVERVIEW 5 FIVE-YEAR SUMMARY 6 FINANCIAL HIGHLIGHTS 7 CEO MESSAGE 8 CORPORATE PROFILE 10 HISTORY 11 BUSINESS MODEL 12 VISION, MISSION AND OBJECTIVES 13 OUR STRATEGY 14 SAMAN FINANCIAL GROUP 16 SHAREHOLDER STRUCTURE AND CAPITAL 18 BUSINESS REVIEW 19 OVERVIEW 20 RETAIL AND ELECTRONIC BANKING 22 INVESTMENT PRODUCTS AND SERVICES 25 INTERNATIONAL BANKING 26 LENDING 28 GOVERNANCE 37 CORPORATE GOVERNANCE 38 BOARD OF DIRECTORS 42 BOARD COMMITTEES 44 INTERNAL AUDIT AND CONTROL 46 INDEPENDENT AUDIT 47 EXECUTIVE MANAGEMENT 48 RISK MANAGEMENT 51 COMPLIANCE 54 HUMAN RESOURCES 56 CORPORATE SOCIAL RESPONSIBILITY 61 FINANCIAL STATEMENTS 67 SAMAN BANK BRANCH NETWORK 99 3 ANNUAL REPORT 2010/11 OVERVIEW 5 ANNUAL REPORT 2010/11 OVERVIEW FIVE-YEAR SUMMARY for the years ended March 21 US$ m Change in % in IRR billion, except where indicated 2011 2011 2010 11/10 2009 2008 2007 Profit and loss data Total income 894 9,262 7,043 31.5 5,700 4,462 2,880 Total expenses 747 7,747 6,164 25.7 5,237 3,950 2,621 Profit before tax 146 1,515 879 72.4 463 512 259 Tax 15 160 92 73.9 24 0 0 Net profit 131 1,355 787 72.2 439 512 258 Balance sheet data Total loans 5,813 60,246 34,184 76.2 27,341 23,989 15,269 Total assets 8,193 84,912 49,315 72.2 41,733 34,846 26,209 Total deposits 5,325 55,189 40,072 37.7 34,167 29,583 22,370 Total liabilities 7,727 80,081 46,393 72.6 39,341 33,320 24,954 Share capital 289 3,000 1,800 66.7 900 900 900 Total shareholders' -
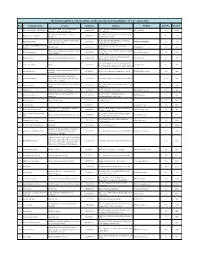
Row Company Name Activity Telephone Address Website Hall
The 6th Int,l. Exhibition of Modern House, Architecture, Interior Design(MIDEX ) -10 to 13 January2016 Row Company Name Activity Telephone Address WebSite Hall No Booth No Smart Home- BMS- Over Air Controlling- No 1., 9th Floor, Tala Building, Second Sadeghie 1 Fan Avaran Khaneh Sabz Kazhal 2144090363 http://boltiran.ir 31A A0101 Building Security- Video Intercom Sq, Tehran, Iran facade design-interior design-landscape- N 6- Shahin building- before tirandaz-tehranpars- 2 Badgir engineer architects 2177724010 31A A02 planning resalat highway- tehran Archietcture Education Institute- Proffessional No.18/10th Alley/GhaemMagham-e- Farahani 3 Naghsh Parsin Hoor 2188170751 WWW.IACENTER.IR 31A A03 Architecture Design Firm St/Beheshti Ave/tehran/Iran ROSHANGARAN ELECTRONIC tehran-abshenasan-golzar sharghi-pelak2- 4 lighting design 2144841261 www.prolight.ae 31A A04 ARAST tabagheh2 office , home and shop interior design . re new no 11 . floor 3 - no 8 . st Rafat . sq Ghods . Tajrish 5 Dorika design home 2122739804 www.dorikadeco.com 31A A05 and diy building . Tehran . Iran unit 11 - no 16 - ramin al - south kazeroun aley - 6 Noorazin nikan Designer and manufacturer of led lighting 2126410826 www.noorazin.ir 31A A07 mirdamad blvd - tehran - iran sarve sepid shiraz Co.,302 St.,North pajoohesh 7 sarve sepid shiraz Import 7137743043 sarvesepid.com 31A B01 St., Pajoohesh Sq., Industrial City, Shiraz, IRAN. consulting , construction and erection of Space 8 stuck pishro sazeh 2177482701 unit 13 , no.3 , dowlat st , pasdaran ave , tehran WWW.3DSpaceco.com -

COVID-19 PCR Test-Customer Version-FA-9
- https://www.qatarairways.com/content/dam/documents/QR-consent-form-PCR.pdf https://www.qatarairways.com/en/travel-alerts/COVID-19-update.html __________________________________________________________________________________________ qatarairways.com/ir THR: 021 75925 MHD: 051 37664101 07 IFN: 031 32353536 SYZ: 071 32271860 9 Dear Customer, Effective13August2020,allpassengerstravellingfromIranonQatarAirwaysflightsare requiredtotakeaRT-PCRtestwithin hourspriordeparture.Thenose/throatswabtest forPCRshouldbedonefromoneofthelaboratoriesauthorizedbyQatarAirwaysin Tehran,Mashhad,ShirazandIsfahan.Negativetestresultsshouldbepresentedatairport atthetimeofcheck-in.Passengersmustpossesstwocopiesofthetestresult,mustfill andsubmitaconsentformalongwiththeRT-PCRtestresult. Download consent form: https://www.qatarairways.com/content/dam/documents/QR- consent-form-PCR.pdf For more information, please visit: https://www.qatarairways.com/en/travel-alerts/COVID- 19-update.html Exemptions: a.Childrenunder12yearsareexemptediftravellingwiththeirfamilywhoareholdinga negativetest. b.InfantsarealsoexemptedfromPCRtestrequirements. Childrenunder12yearstravellingasUM(unaccompaniedminor)arenotexemptedand mustholdcertificateofprooffromtheauthorizedmedicalcenter. Approved labs are listed in the following page. Thank you. Qatar Airways __________________________________________________________________________________________ qatarairways.com/ir THR: 021 75925 MHD: 051 37664101 07 IFN: 031 32353536 SYZ: 071 32271860 9 Tehran Lab Name Telephone Address Gholhak Lab 22600413 -

Department of the Treasury
Vol. 81 Monday, No. 49 March 14, 2016 Part IV Department of the Treasury Office of Foreign Assets Control Changes to Sanctions Lists Administered by the Office of Foreign Assets Control on Implementation Day Under the Joint Comprehensive Plan of Action; Notice VerDate Sep<11>2014 14:39 Mar 11, 2016 Jkt 238001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\14MRN2.SGM 14MRN2 jstallworth on DSK7TPTVN1PROD with NOTICES 13562 Federal Register / Vol. 81, No. 49 / Monday, March 14, 2016 / Notices DEPARTMENT OF THE TREASURY Department of the Treasury (not toll free Individuals numbers). 1. AFZALI, Ali, c/o Bank Mellat, Tehran, Office of Foreign Assets Control SUPPLEMENTARY INFORMATION: Iran; DOB 01 Jul 1967; nationality Iran; Electronic and Facsimile Availability Additional Sanctions Information—Subject Changes to Sanctions Lists to Secondary Sanctions (individual) Administered by the Office of Foreign The SDN List, the FSE List, the NS– [NPWMD] [IFSR]. Assets Control on Implementation Day ISA List, the E.O. 13599 List, and 2. AGHA–JANI, Dawood (a.k.a. Under the Joint Comprehensive Plan additional information concerning the AGHAJANI, Davood; a.k.a. AGHAJANI, of Action JCPOA and OFAC sanctions programs Davoud; a.k.a. AGHAJANI, Davud; a.k.a. are available from OFAC’s Web site AGHAJANI, Kalkhoran Davood; a.k.a. AGENCY: Office of Foreign Assets AQAJANI KHAMENA, Da’ud); DOB 23 Apr (www.treas.gov/ofac). Certain general Control, Treasury Department. 1957; POB Ardebil, Iran; nationality Iran; information pertaining to OFAC’s Additional Sanctions Information—Subject ACTION: Notice. sanctions programs is also available via to Secondary Sanctions; Passport I5824769 facsimile through a 24-hour fax-on- (Iran) (individual) [NPWMD] [IFSR]. -

SDN Changes 2017
OFFICE OF FOREIGN ASSETS CONTROL CHANGES TO THE Specially Designated Nationals and Blocked Persons List SINCE JANUARY 1, 2017 This publication of Treasury's Office of Foreign AL-BANNA, Ibrahim (a.k.a. AL-BANNA, Ibrahim BELMEX IMPORT EXPORT CO., LTD., 24 Assets Control ("OFAC") is designed as a Muhamad Salih; a.k.a. AL-BANNA, Ibrahim Corner Regent and Kings Streets, Belize City, reference tool providing actual notice of actions by Muhammad Salih; a.k.a. AL-BANNA, Shaykh Belize [CUBA]. OFAC with respect to Specially Designated Ibrahim Muhammad Salih; a.k.a. AL-MASRI, CARIBSUGAR INTERNATIONAL TRADERS, Nationals and other entities whose property is Abu Ayman); DOB 1965; nationality Egypt; S.A., 125-133 Camden High Street, London blocked, to assist the p ublic in complying with the Gender Male (individual) [SDGT]. NW1 7JR, United Kingdom [CUBA]. various sanctions programs administered by AL-BANNA, Ibrahim Muhamad Salih (a.k.a. AL- COLONY TRADING, S.A., Panama [CUBA]. OFAC. The latest changes may appear here prior BANNA, Ibrahim; a.k.a. AL-BANNA, Ibrahim COPIA, S.A. (a.k.a. CORPORACION to their publication in the Federal Register, and it Muhammad Salih; a.k.a. AL-BANNA, Shaykh ARGENTINA DE INGENIERIA Y is intended that users rely on changes indicated in Ibrahim Muhammad Salih; a.k.a. AL-MASRI, ARQUITECTURA, S.A.), San Martin 323, 4th this document that post -date the most recent Abu Ayman); DOB 1965; nationality Egypt; Floor, Buenos Aires, Argentina [CUBA]. Federal Register publication with respect to a Gender Male (individual) [SDGT]. CORPORACION ARGENTINA DE INGENIERIA Y particular sanctions program in the appendices to AL-BANNA, Ibrahim Muhammad Salih (a.k.a.