The Board of Regents of the University of Wisconsin System Project
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

June 2016 1 THINGS to DO / THINGS to THINK ABOUT THIS MONTH
Spring/early summer is here and the 2016 growing season is well underway. The predictable unpredictability of the weather has brought the typical challenges that we face on an annual basis. Hopefully the majority of you are untouched or unaffected by the quirky weather that has been observed. Hopefully you’ve gotten some of the rain that has fallen across the Prairies. To combat or counteract the unpredictability of the weather, welcome to another ever-so-predictable edition of Hort Snacks. The content isn’t always predictable, but the arrival of it is. In this edition, you’ll notice a theme. And that theme is largely creepy/crawly. The season of insect wildlife is upon us, and to prepare you (and perhaps some of your customers) for identifying and dealing with some of those pests, you’ll find some caterpillar-related resources. That doesn’t mean that there aren’t more things out there, but this is a start. Otherwise, please take a moment to look at the various bits and pieces that might be of interest to you. Regardless, we wish you all the best for your growing season. Stay in touch. Rob Spencer/Dustin Morton, Commercial Horticulture Specialists Alberta Ag-Info Centre Alberta Agriculture and Forestry 310-FARM (3276) In this edition of Hort Snacks . Featured Surveys ………1 . Things to Do / Things to Think About ………2 . Interesting News/Articles to Read ………2 FEATURED SURVEYS . DED Awareness Week Reminder ………2 . Upcoming Conferences/Workshops ………3 . Input being sought with regards to Future Government Hort Snacks in the Field - Veg & Fruit Field Days ………3 . -
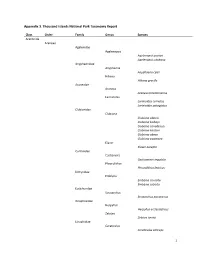
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Moth Checklist Pagemaker
thePembrokeAreaFieldNaturalists presents a MothChecklist of central Renfrew County, Ontario compiled by Jason J. Dombroskie ©chris michener February 2003 Checklist of Moths of the Pembroke Area Field Naturalists Compiled by Jason J. Dombroskie. Status: VR = very rare, R = rare, U = uncommon, C = common Scientific Name Common Name status Family Hepialidae Ghost Moths Sthenopis argenteomaculatus (Harris) Silver-spotted Ghost Moth U Sthenopis quadriguttatus (Grote) Four-spotted Ghost Moth U Family Nepticulidae Minute Eye-cap Moths Ectoedemia populella Busck Poplar Petiolegall Moth C Ectoedemia ulmella (Braun) C Glaucolepis saccharella (Braun) Stigmella corylifoliella (Clemens) U Stigmella saginella (Clemens) R Family Heliozelidae Shield Bearer Moths Antispila freemani Lafontaine R Family Adelidae Fairy Moths Adela purpurea Walker Willow Fairy Moth U Family Incurvariidae Leafcutter Moths Paraclemensia acerifoliella (Fitch) Maple Leafcutter C Family Tineidae Fungus Moths Eccritothrix trimaculella (Chambers) R Nemapogon granella (Linnaeus) R Family Gracillariidae Leaf Miner Moths Caloptilia fraxinella (Ely) Privet Leaf Miner C Caloptilia invariabilis (Braun) Cherry Leafcone Caterpillar C Caloptilia near stigmatella (Fabricus) C Caloptilia umbratella (Braun) R Parornix crataegifoliella (Clemens) R Parornix inusitatumella (Chambers) R Phyllonorycter aeriferella (Clemens) R Phyllonorycter argentifimbriella (Clemens) R Phyllonorycter basistrigella (Clemens) R Phyllonorycter lucetiella (Clemens) Basswood Squareblotch Miner U Phyllonorycter ostryaefoliella -

Moths of North Carolina - Early Draft 1
Noctuidae Achatia distincta Distinct Quaker Moth 20 n=12 • • High Mt. • • • • N 10 • •• u • • • m • • • • b • 0 • • e • • • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 • 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 27 • Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec • o 20 • f n=39 • = Sighting or Collection Low Mt. High counts of: in NC since 2001 F • = Not seen since 2001 l 10 30 - Ashe - 2000-05-02 • i 8 - Macon - 2001-04-21 g Status Rank h 6 - Ashe - 2000-05-02 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 20 20 t n=35 n=7 e Pd CP s 10 10 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Noctuidae SUBFAMILY: Noctuinae TRIBE: Orthosiini TAXONOMIC_COMMENTS: A monotypic genus found across most of eastern North America and throughout North Carolina. -

Australia's Biodiversity and Climate Change
Australia’s Biodiversity and Climate Change A strategic assessment of the vulnerability of Australia’s biodiversity to climate change A report to the Natural Resource Management Ministerial Council commissioned by the Australian Government. Prepared by the Biodiversity and Climate Change Expert Advisory Group: Will Steffen, Andrew A Burbidge, Lesley Hughes, Roger Kitching, David Lindenmayer, Warren Musgrave, Mark Stafford Smith and Patricia A Werner © Commonwealth of Australia 2009 ISBN 978-1-921298-67-7 Published in pre-publication form as a non-printable PDF at www.climatechange.gov.au by the Department of Climate Change. It will be published in hard copy by CSIRO publishing. For more information please email [email protected] This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Commonwealth. Requests and inquiries concerning reproduction and rights should be addressed to the: Commonwealth Copyright Administration Attorney-General's Department 3-5 National Circuit BARTON ACT 2600 Email: [email protected] Or online at: http://www.ag.gov.au Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for Climate Change and Water and the Minister for the Environment, Heritage and the Arts. Citation The book should be cited as: Steffen W, Burbidge AA, Hughes L, Kitching R, Lindenmayer D, Musgrave W, Stafford Smith M and Werner PA (2009) Australia’s biodiversity and climate change: a strategic assessment of the vulnerability of Australia’s biodiversity to climate change. -

Contributions Toward a Lepidoptera (Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, Thyrididae, Drepanoidea, Geometro
Contributions Toward a Lepidoptera (Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, Thyrididae, Drepanoidea, Geometroidea, Mimalonoidea, Bombycoidea, Sphingoidea, & Noctuoidea) Biodiversity Inventory of the University of Florida Natural Area Teaching Lab Hugo L. Kons Jr. Last Update: June 2001 Abstract A systematic check list of 489 species of Lepidoptera collected in the University of Florida Natural Area Teaching Lab is presented, including 464 species in the superfamilies Drepanoidea, Geometroidea, Mimalonoidea, Bombycoidea, Sphingoidea, and Noctuoidea. Taxa recorded in Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, and Thyrididae are also included. Moth taxa were collected at ultraviolet lights, bait, introduced Bahiagrass (Paspalum notatum), and by netting specimens. A list of taxa recorded feeding on P. notatum is presented. Introduction The University of Florida Natural Area Teaching Laboratory (NATL) contains 40 acres of natural habitats maintained for scientific research, conservation, and teaching purposes. Habitat types present include hammock, upland pine, disturbed open field, cat tail marsh, and shallow pond. An active management plan has been developed for this area, including prescribed burning to restore the upland pine community and establishment of plots to study succession (http://csssrvr.entnem.ufl.edu/~walker/natl.htm). The site is a popular collecting locality for student and scientific collections. The author has done extensive collecting and field work at NATL, and two previous reports have resulted from this work, including: a biodiversity inventory of the butterflies (Lepidoptera: Hesperioidea & Papilionoidea) of NATL (Kons 1999), and an ecological study of Hermeuptychia hermes (F.) and Megisto cymela (Cram.) in NATL habitats (Kons 1998). Other workers have posted NATL check lists for Ichneumonidae, Sphecidae, Tettigoniidae, and Gryllidae (http://csssrvr.entnem.ufl.edu/~walker/insect.htm). -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -

Insects That Feed on Trees and Shrubs
INSECTS THAT FEED ON COLORADO TREES AND SHRUBS1 Whitney Cranshaw David Leatherman Boris Kondratieff Bulletin 506A TABLE OF CONTENTS DEFOLIATORS .................................................... 8 Leaf Feeding Caterpillars .............................................. 8 Cecropia Moth ................................................ 8 Polyphemus Moth ............................................. 9 Nevada Buck Moth ............................................. 9 Pandora Moth ............................................... 10 Io Moth .................................................... 10 Fall Webworm ............................................... 11 Tiger Moth ................................................. 12 American Dagger Moth ......................................... 13 Redhumped Caterpillar ......................................... 13 Achemon Sphinx ............................................. 14 Table 1. Common sphinx moths of Colorado .......................... 14 Douglas-fir Tussock Moth ....................................... 15 1. Whitney Cranshaw, Colorado State University Cooperative Extension etnomologist and associate professor, entomology; David Leatherman, entomologist, Colorado State Forest Service; Boris Kondratieff, associate professor, entomology. 8/93. ©Colorado State University Cooperative Extension. 1994. For more information, contact your county Cooperative Extension office. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, -

Coleoptera, Curculionidae, Entiminae)
Machado_Pruebas 27/12/10 12:51 Página 233 Graellsia, 66(2): 233-280 julio-diciembre 2010 ISSN: 0367-5041 doi:10.3989/graellsia.2010.v66.025 LA MORFOLOGÍA DE LAPAROCERUS UNDATUS WOLLASTON, 1864 Y CONSIDERACIONES SOBRE LA TRIBU LAPAROCERINI LACORDAIRE, 1863 (COLEOPTERA, CURCULIONIDAE, ENTIMINAE) A. Machado* RESUMEN A. Machado. 2010. La morfología de Laparocerus undatus Wollaston, 1864 y consideracio- nes sobre la tribu Laparocerini Lacordaire, 1863 (Coleoptera, Curculionidae, Entiminae). Graellsia, 66(2): 233-280. Se presenta un estudio morfológico de Laparocerus undatus Wollaston, 1864 que com- prende la anatomía externa e interna del adulto, incluido el canal alimentario, sistema ner- vioso central y sistema reproductor. También se describen las anatomías de la larva y de la pupa, hasta ahora desconocidas en el género. Los Laparocerus son curculiónidos entimi- nos que muestran algunos caracteres primitivos infrecuentes en el grupo. Se plantea si se justifica o no mantener la tribu Laparocerini, pendiente de revisión desde que fuera esta- blecida por Lacordaire en 1863. Se discuten las semejanzas halladas en los caracteres ima- ginales y preimaginales con otros géneros y tribus, y se buscan las relaciones genéticas más cercanas empleando secuencias parciales del gen 16SrRNA de una veintena de espe- cies, obtenidas de GenBank. Laparocerus presenta importantes caracteres singulares o combinación de ellos, y se separa claramente de los demás Entiminae estudiados, ocupan- do una posición posiblemente basal dentro del grupo. Se proponen algunos -

Stress Test: Effects of Endosymbiotic Bacteria on Thermal Tolerance of a Montane Leaf
Stress test: Effects of endosymbiotic bacteria on thermal tolerance of a montane leaf beetle By Bo Zhang A thesis submitted to Sonoma State University In partial fulfillment of the requirements For the degree of Master of Science In Biology Dr. Nathan Rank, Chair Dr. Elizabeth Dahlhoff Dr. Sean Place June 29, 2018 i Copyright 2018 By Bo Zhang ii Authorization for Reproduction of Master’s Thesis/Project I grant permission for the print or digital reproduction of this thesis in its entirety, without further authorization from me, on the condition that the person or agency requesting reproduction absorb the cost and provide proper acknowledgement of authorship. Date: June 29, 2018 Bo Zhang iii Stress test: Effects of endosymbiotic bacteria on thermal tolerance of a montane leaf beetle Thesis by Bo Zhang Abstract Insects are a diverse group of organisms found in most terrestrial and aquatic ecosystems. Because insects are ectotherms, they are frequently exposed to variation in environmental temperature and thus variation in body temperature, the latter of which may cause physiological stress. Insects host a variety of microbes, including intracellular and extracellular bacteria, endosymbiotic or parasitic, which may alter how the host responds to environmental temperature variation. Physiological, biochemical and molecular responses to thermal variation have been well characterized for many species of insects, yet few studies have examined how microbes alter their response to environmental stress. In this thesis, I examined features of the microbiome of a montane insect, the leaf beetle Chrysomela aeneicollis. Prior studies of beetle populations living at high elevation in the Eastern Sierra Nevada mountains of California have shown that adult and larval beetles are exposed to both elevated and sub-zero temperatures during the summer growing season, and that exposure to these thermal extremes impacts survival, performance, and reproductive success. -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties.