Downloaded on 20 April 2011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORNITHOLOGIST VOLUME 44 - PARTS 1&2 - November - 2019
SOUTH AUSTRALIAN ORNITHOLOGIST VOLUME 44 - PARTS 1&2 - November - 2019 Journal of The South Australian Ornithological Association Inc. In this issue: Variation in songs of the White-eared Honeyeater Phenotypic diversity in the Copperback Quailthrush and a third subspecies Neonicotinoid insecticides Bird Report, 2011-2015: Part 1, Non-passerines President: John Gitsham The South Australian Vice-Presidents: Ornithological John Hatch, Jeff Groves Association Inc. Secretary: Kate Buckley (Birds SA) Treasurer: John Spiers FOUNDED 1899 Journal Editor: Merilyn Browne Birds SA is the trading name of The South Australian Ornithological Association Inc. Editorial Board: Merilyn Browne, Graham Carpenter, John Hatch The principal aims of the Association are to promote the study and conservation of Australian birds, to disseminate the results Manuscripts to: of research into all aspects of bird life, and [email protected] to encourage bird watching as a leisure activity. SAOA subscriptions (e-publications only): Single member $45 The South Australian Ornithologist is supplied to Family $55 all members and subscribers, and is published Student member twice a year. In addition, a quarterly Newsletter (full time Student) $10 reports on the activities of the Association, Add $20 to each subscription for printed announces its programs and includes items of copies of the Journal and The Birder (Birds SA general interest. newsletter) Journal only: Meetings are held at 7.45 pm on the last Australia $35 Friday of each month (except December when Overseas AU$35 there is no meeting) in the Charles Hawker Conference Centre, Waite Road, Urrbrae (near SAOA Memberships: the Hartley Road roundabout). Meetings SAOA c/o South Australian Museum, feature presentations on topics of ornithological North Terrace, Adelaide interest. -

Heritage of the Birdsville and Strzelecki Tracks
Department for Environment and Heritage Heritage of the Birdsville and Strzelecki Tracks Part of the Far North & Far West Region (Region 13) Historical Research Pty Ltd Adelaide in association with Austral Archaeology Pty Ltd Lyn Leader-Elliott Iris Iwanicki December 2002 Frontispiece Woolshed, Cordillo Downs Station (SHP:009) The Birdsville & Strzelecki Tracks Heritage Survey was financed by the South Australian Government (through the State Heritage Fund) and the Commonwealth of Australia (through the Australian Heritage Commission). It was carried out by heritage consultants Historical Research Pty Ltd, in association with Austral Archaeology Pty Ltd, Lyn Leader-Elliott and Iris Iwanicki between April 2001 and December 2002. The views expressed in this publication are not necessarily those of the South Australian Government or the Commonwealth of Australia and they do not accept responsibility for any advice or information in relation to this material. All recommendations are the opinions of the heritage consultants Historical Research Pty Ltd (or their subconsultants) and may not necessarily be acted upon by the State Heritage Authority or the Australian Heritage Commission. Information presented in this document may be copied for non-commercial purposes including for personal or educational uses. Reproduction for purposes other than those given above requires written permission from the South Australian Government or the Commonwealth of Australia. Requests and enquiries should be addressed to either the Manager, Heritage Branch, Department for Environment and Heritage, GPO Box 1047, Adelaide, SA, 5001, or email [email protected], or the Manager, Copyright Services, Info Access, GPO Box 1920, Canberra, ACT, 2601, or email [email protected]. -

Mailman of the Birdsville Track: the Story of Tom Kruse Free
FREE MAILMAN OF THE BIRDSVILLE TRACK: THE STORY OF TOM KRUSE PDF Kristin Weidenbach,Peter Hosking | none | 11 Mar 2013 | Bolinda Publishing | 9781743156735 | English | Australia Tom Kruse the birdsville mailman Goodreads helps you keep track of Mailman of the Birdsville Track: The Story of Tom Kruse you want to read. Want to Read saving…. Want to Read Currently Reading Read. Other editions. Enlarge cover. Error rating book. Refresh and try again. Open Preview See a Problem? Details if other :. Thanks for telling us about the problem. Return to Book Page. The Birdsville Track is one of the best-known tracks in Australia, and for 20 years Tom Kruse was the mailman, battling searing heat, floods Mailman of the Birdsville Track: The Story of Tom Kruse mechanical breakdowns. He made the run every fortnight and was a lifeline to the isolated settlements and stations along the way, delivering everything from letters to essential supplies. Get A Copy. Paperbackpages. Published by Hodder first published January More Details Original Title. Other Editions Friend Reviews. To see what your friends thought of this book, please sign up. To ask other readers questions about Mailman of the Birdsville Trackplease sign up. Be the first to ask a question about Mailman of the Birdsville Track. Lists with This Book. Community Reviews. Showing Average rating 3. Rating details. More filters. Sort order. Jan 22, Janelle marked it as dnf. I didn't get far into the first chapter. Maybe I'm too much of an impatient reader, but I was very bored. View all 3 comments. Jan 08, Dale rated it did not like it Shelves: abandoned. -

On Track 2010-2011
ON TRACK Delivering NRM in the SA Arid Lands 2010-11 ON TRACK Delivering natural resources management in the SA Arid Lands 2010-11 Protecting our land, plants and animals Understanding and securing our water resources Supporting our industries and communities 1 Welcome It is with great pleasure that I introduce this first edition of On Track. Having now completed the first year the achievements of former Presiding of delivery of the South Australian Arid Member Chris Reed, previous members Lands (SAAL) Regional Natural Resources of the Board, and General Manager John Management (NRM) Plan which sets the Gavin. Almost all of the activities you will direction for natural resources management read about here were initiated through their in the region to 2020, On Track is a report efforts and the current Board is building on to our community on the progress we made their endeavours. in 2010-11 on meeting the Plan’s targets. This year was also marked by the True to the SAAL NRM Board’s platform establishment of the new Department of and the spirit of natural resources Environment and Natural Resources in July management, On Track’s focus is on 2010 which brings together staff from the community. Outback office of the former Department We showcase the variety of projects and for Environment and Heritage and the staff activities where community members are of the SAAL NRM Board. working with the Board. This new integrated service will use a We share with you the experiences of landscape approach to manage natural some of the landholders and community resources across public and private land members involved with our programs and provide a single face for environment including Ecosystem Management and natural resources services in our Understanding™, Pest Management and region. -

National Recovery Plan for the Plains Mouse Pseudomys Australis 2012
National Recovery Plan for the Plains Mouse Pseudomys australis 2012 - 1 - This plan should be cited as follows: Moseby, K. (2012) National Recovery Plan for the Plains Mouse Pseudomys australis. Department of Environment, Water and Natural Resources, South Australia. Published by the Department of Environment, Water and Natural Resources, South Australia. Adopted under the Environment Protection and Biodiversity Conservation Act 1999: [date to be supplied] ISBN : 978-0-9806503-1-0 © Department of Environment, Water and Natural Resources, South Australia. This publication is copyright. Apart from any use permitted under the copyright Act 1968, no part may be reproduced by any process without prior written permission from the Government of South Australia. Requests and inquiries regarding reproduction should be addressed to: Department of Environment, Water and Natural Resources GPO Box 1047 ADELAIDE SA 5001 Note: This recovery plan sets out the actions necessary to stop the decline of, and support the recovery of, the listed threatened species or ecological community. The Australian Government is committed to acting in accordance with the plan and to implementing the plan as it applies to Commonwealth areas. The plan has been developed with the involvement and cooperation of a broad range of stakeholders, but individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge. Queensland disclaimer: The Australian Government, in partnership with the Queensland Department of Environment and Heritage Protection, facilitates the publication of recovery plans to detail the actions needed for the conservation of threatened native wildlife. -
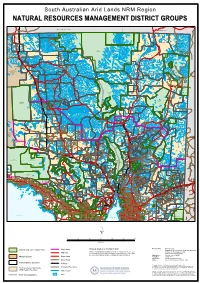
Natural Resources Management District Groups
South Australian Arid Lands NRM Region NNAATTUURRAALL RREESSOOUURRCCEESS MMAANNAAGGEEMMEENNTT DDIISSTTRRIICCTT GGRROOUUPPSS NORTHERN TERRITORY QUEENSLAND Mount Dare H.S. CROWN POINT Pandie Pandie HS AYERS SIMPSON DESERT RANGE SOUTH Tieyon H.S. CONSERVATION PARK ALTON DOWNS TIEYON WITJIRA NATIONAL PARK PANDIE PANDIE CORDILLO DOWNS HAMILTON DEROSE HILL Hamilton H.S. SIMPSON DESERT KENMORE REGIONAL RESERVE Cordillo Downs HS PARK Lambina H.S. Mount Sarah H.S. MOUNT Granite Downs H.S. SARAH Indulkana LAMBINA Todmorden H.S. MACUMBA CLIFTON HILLS GRANITE DOWNS TODMORDEN COONGIE LAKES Marla NATIONAL PARK Mintabie EVERARD PARK Welbourn Hill H.S. WELBOURN HILL Marla - Oodnadatta INNAMINCKA ANANGU COWARIE REGIONAL PITJANTJATJARAKU Oodnadatta RESERVE ABORIGINAL LAND ALLANDALE Marree - Innamincka Wintinna HS WINTINNA KALAMURINA Innamincka ARCKARINGA Algebuckinna Arckaringa HS MUNGERANIE EVELYN Mungeranie HS DOWNS GIDGEALPA THE PEAKE Moomba Evelyn Downs HS Mount Barry HS MOUNT BARRY Mulka HS NILPINNA MULKA LAKE EYRE NATIONAL MOUNT WILLOUGHBY Nilpinna HS PARK MERTY MERTY Etadunna HS STRZELECKI ELLIOT PRICE REGIONAL CONSERVATION ETADUNNA TALLARINGA PARK RESERVE CONSERVATION Mount Clarence HS PARK COOBER PEDY COMMONAGE William Creek BOLLARDS LAGOON Coober Pedy ANNA CREEK Dulkaninna HS MABEL CREEK DULKANINNA MOUNT CLARENCE Lindon HS Muloorina HS LINDON MULOORINA CLAYTON Curdimurka MURNPEOWIE INGOMAR FINNISS STUARTS CREEK SPRINGS MARREE ABORIGINAL Ingomar HS LAND CALLANNA Marree MUNDOWDNA LAKE CALLABONNA COMMONWEALTH HILL FOSSIL MCDOUAL RESERVE PEAK Mobella -

M01: Mineral Exploration Licence Applications
M01 Mineral Exploration Licence Applications 27 September 2021 Resources and Energy Group L4 11 Waymouth Street, Adelaide SA 5000 http://energymining.sa.gov.au/minerals GPO Box 320, ADELAIDE, SA 5001 http://energymining.sa.gov.au/energy_resources Phone +61 8 8463 3000 http://map.sarig.sa.gov.au Email [email protected] Earth Resources Information Sheet - M1 Printed on: 27/09/2021 M01: Mineral Exploration Licence Applications Year Lodged: 1996 File Ref. Applicant Locality Fee Zone Area (km2 ) 250K Map 1996/00118 NiCul Minerals Limited Mount Harcus area - Approx 400km 2,415 Lindsay, WNW of Marla Woodroffe 1996/00185 NiCul Minerals Limited Willugudinna Hill area - Approx 823 Everard 50km NW of Marla 1996/00260 Goldsearch Ltd Ernabella South area - Approx 519 Alberga 180km NW of Marla 1996/00262 Goldsearch Ltd Marble Hill area - Approx 80km NW 463 Abminga, of Marla Alberga 1996/00340 Goldsearch Ltd Birksgate Range area - Approx 2,198 Birksgate 380km W of Marla 1996/00341 Goldsearch Ltd Ayliffe Hill area - Approx 220km NW 1,230 Woodroffe of Marla 1996/00342 Goldsearch Ltd Musgrave Ranges area - Approx 2,136 Alberga 180km NW of Marla 1996/00534 Caytale Pty Ltd Bull Hill area - Approx 240km NW of 1,783 Woodroffe Marla Year Lodged: 1997 File Ref. Applicant Locality Fee Zone Area (km2 ) 250K Map 1997/00040 Minex (Aust) Pty Ltd Bowden Hill area - Approx 300 WNW 1,507 Woodroffe of Marla 1997/00053 Mithril Resources Limited Mt Cooperina Area - approx. 360 km 1,013 Mann WNW of Marla 1997/00055 Mithril Resources Limited Oonmooninna Hill Area - approx. -

MARREE - INNAMINCKA Natural Resources Management Group
South Australian Arid Lands NRM Region MARREE - INNAMINCKA Natural Resources Management Group NORTHERN TERRITORY QUEENSLAND SIMPSON DESERT CONSERVATION PARK Pastoral Station ALTON DOWNS MULKA PANDIE PANDIE Boundary CORDILLO DOWNS Conservation and National Parks Regional reserve/ SIMPSON DESERT Pastoral Station REGIONAL RESERVE Aboriginal Land Marree - Innamincka CLIFTON HILLS NRM Group COONGIE LAKES NATIONAL PARK INNAMINCKA REGIONAL RESERVE SA Arid Lands NRM Region Boundary INNAMINCKA Dog Fence COWARIE Major Road MACUMBA ! KALAMURINA Innamincka Minor Road / Track MUNGERANIE Railway GIDGEALPA ! Moomba Cadastral Boundary THE PEAKE Watercourse LAKE EYRE (NORTH) LAKE EYRE MULKA Mainly Dry Lake NATIONAL PARK MERTY MERTY STRZELECKI ELLIOT PRICE REGIONAL CONSERVATION RESERVE PARK ETADUNNA BOLLARDS ANNA CREEK LAGOON DULKANINNA MULOORINA LINDON LAKE BLANCHE LAKE EYRE (SOUTH) MULOORINA CLAYTON MURNPEOWIE Produced by: Resource Information, Department of Water, Curdimurka ! STRZELECKI Land and Biodiversity Conservation. REGIONAL Data source: Pastoral lease names and boundaries supplied by FINNISS MARREE RESERVE Pastoral Program, DWLBC. Cadastre and Reserves SPRINGS LAKE supplied by the Department for Environment and CALLANNA ABORIGINAL ! Marree CALLABONNA Heritage. Waterbodies, Roads and Place names LAND FOSSIL supplied by Geoscience Australia. STUARTS CREEK MUNDOWDNA Projection: MGA Zone 53. RESERVE Datum: Geocentric Datum of Australia, 1994. MOOLAWATANA MOUNT MOUNT LYNDHURST FREELING FARINA MULGARIA WITCHELINA UMBERATANA ARKAROOLA WALES SOUTH NEW -

Regional-Map-Outback-Qld-Ed-6-Back
Camooweal 160 km Burke and Wills Porcupine Gorge Charters New Victoria Bowen 138° Camooweal 139° 140° 141° Quarrells 142° 143° Marine fossil museum, Compton Downs 144° 145° 146° Charters 147° Burdekin Bowen Scottville 148° Roadhouse 156km Harrogate NP 18 km Towers Towers Downs 80 km 1 80 km 2 3 West 4 5 6 Kronosaurus Korner, and 7 8 WHITE MTNS Warrigal 9 Milray 10 Falls Dam 11 George Fisher Mine 139 OVERLANDERS 48 Nelia 110 km 52 km Harvest Cranbourne 30 Leichhardt 14 18 4 149 recreational lake. 54 Warrigal Cape Mt Raglan Collinsville Lake 30 21 Nonda Home Kaampa 18 Torver 62 Glendower NAT PARK 14 Biralee INDEX OF OUTBACK TOWNS AND Moondarra Mary Maxwelton 32 Alston Vale Valley C Corea Mt Malakoff Mt Bellevue Glendon Heidelberg CLONCURRY OORINDI Julia Creek 57 Gemoka RICHMOND Birralee 16 Tom’s Mt Kathleen Copper and Gold 9 16 50 Oorindi Gilliat FLINDERS A 6 Gypsum HWY Lauderdale 81 Plains LOCALITIES WITH FACILITIES 11 18 9THE Undha Bookin Tibarri 20 Rokeby 29 Blantyre Torrens Creek Victoria Downs BARKLY 28 Gem Site 55 44 Marathon Dunluce Burra Lornsleigh River Gem Site JULIA Bodell 9 Alick HWY Boree 30 44 A 6 MOUNT ISA BARKLY HWY Oonoomurra Pymurra 49 WAY 23 27 HUGHENDEN 89 THE OVERLANDERS WAY Pajingo 19 Mt McConnell TENNIAL River Creek A 2 Dolomite 35 32 Eurunga Marimo Arrolla Moselle 115 66 43 FLINDERS NAT TRAIL Section 3 Outback @ Isa Explorers’ Park interprets the World Rose 2 Torrens 31 Mt Michael Mica Creek Malvie Downs 52 O'Connell Warreah 20 Lake Moocha Lake Ukalunda Mt Ely A Historic Cloncurry Shire Hall, 25 Rupert Heritage listed Riversleigh Fossil Field and has underground mine tours. -

Outback South Australia & Flinders
A B C Alice Springs D E F G H J K Kulgera QAA Big Red Surveyor NORTHERN TERRITORY NORTHERN TERRITORY LINE Generals Poeppel Corner SOUTH AUSTRALIA LINE Birdsville QUEENSLAND Haddon Corner Major Road Sealed K1 Corner SOUTH AUSTRALIA Mount Dare Hotel SOUTH AUSTRALIA Witjira National Park FRENCH Major Road Unsealed RIG Simpson Desert Mt Woodroffe Dalhousie Conservation Park 1 Springs YANDRUWANDHA 1 Secondary Road Sealed RIG / YAWARRAWARRKA RD RD Secondary Road Unsealed LINE Aparawatatja Strzelecki Community Alberga Goyder 'Cordillo Downs' Other Road Unsealed Fregon WANGKANGURRU / YARLULANDI Lagoon Desert Simpson Desert 'Arrabury' 4WD Only Simpson Desert River Macumba Innamincka Station ANANGU Regional Reserve Regional PITJANTJATJARA Warburton Marla OODNADATTA Reserve YANKUNYTJATJARA Mintabie Crossing Coongie Lakes Explorer’s Way STUART River National Park WESTERN ABORIGINAL Ck Sturt LAND A87 Route Marker Oodnadatta Ck 2 ANTAKIRIJA 2 Stony Walkers Crossing Visitor Information Centre ANANGU PITJANTJATJARA RD 'Kalamurina' RD River Warburton Innamincka YANKUNYTJATJARA Cadney DESERT Desert Aboriginal Cultural Experience PAINTED Homestead TRACK 'Copper Accommodation Hills' KEMPE Mungerannie (Indicated for Outback and Neales Hotel Moomba Flinders Ranges region only) Lake Eyre (No Public SOUTH Great Victoria Desert Tirari Services) Mamungari Con. Park Lake Eyre Cooper Annes Corner Defence North National Park Desert Centre A87 ANNE Tallaringa Elliot TRACK Strzelecki Vokes Hill Woomera Conservation Corner MARALINGA Price Strzelecki Park William QUEENSLAND TJARUTJA THE Con. Lake 3 Creek Regional Desert SOUTH AUSTRALIA 3 ABORIGINAL BEADELL Gregory HWY Park DIERI Reserve LAND Coober Pedy RD See Dog Fence WILLIAM CREEK PASTORAL PROPERTIES Lake Eyre South Outback Cameron The roads in this region pass through working ARABUNNA TRACK 'Muloorina' BIRDSVILLE Corner pastoral properties. -

Data on Significant Wilderness Areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions
Data on significant wilderness areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions Wilderness Advisory Committee November 2014 Acknowledgments The Wilderness Advisory Committee acknowledges the invaluable work of the late Dr Rob Lesslie. His work forms the basis of much of this report, with the Wilderness Advisory Committee holding responsibility for the report. We thank the staff of the Department of Environment, Water and Natural Resources for their assistance, in particular Jason Irving and Ian Sellar. i | Data on significant wilderness areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions Contents 1. Purpose of the report 1 2. The significance of wilderness 1 3. Wilderness surveys 3 4. Adequacy of formal protection 3 5. Management principles for the arid environment 4 6. Conclusion 5 Appendix 1. Wilderness Areas of Potential 6 National Significance: description Appendix 2. Climate change priority actions 26 Appendix 3 Maps 28 Map 1 Wilderness Areas of Potential 29 National Significance: Bioregions Map 2 Wilderness Areas of Potential 31 National Significance: Land Ownership Map 3 Wilderness Areas of Potential 33 National Significance: Watercourses and wetlands Map 4 Wilderness Areas of Potential 35 National Significance: Waterpoints Map 5 National Wilderness Inventory 37 Map 6 Wilderness Areas of Potential 39 National Significance: Conservation Area Type Map 7 Bioregional Distribution of Highly 41 Protected Areas (IUCN Category Ia, Ib, II and III) Map 8 Predicted Temperature Increase 42 for South Australia, 2030, 2050 and 2070 Data on significant wilderness areas in the Alinytjara Wilurara and South Australian Arid Lands NRM Regions | ii Left and right image: Nullabor Plains, South Australia 1. -

Integrated Analysis of the Movement and Ecology of Wild Dingoes in The
Integrated analysis of the movement and ecology of wild dingoes in the arid zone Jack Tatler A thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy April 2019 School of Biological Sciences The University of Adelaide, South Australia Table of Contents Abstract ...................................................................................................................................................... i Thesis Declaration .................................................................................................................................... iii Acknowledgements .................................................................................................................................. iv Chapter 1. Introduction ............................................................................................................................. 1 Chapter 2. Phenotypic variation in coat colour and mating strategy in a wild population of pure dingoes Canis dingo................................................................................................................................... 9 Chapter 3. Resource pulses affect prey selection and reduce dietary diversity of dingoes in arid Australia .................................................................................................................................................. 32 Chapter 4. High accuracy at low frequency: detailed behavioural classification from accelerometer data ........................................................................................................................................................