<I>Coccostromopsis Palmicola</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Syagrus Romanzoffiana [Cham.] Glassman](https://docslib.b-cdn.net/cover/5715/syagrus-romanzoffiana-cham-glassman-115715.webp)
Syagrus Romanzoffiana [Cham.] Glassman
SCIENTIFIC note Doi: https://doi.org/10.17584/rcch.2019v13i3.8363 Pre-depulping and depulping treatments and the emergence of queen palm seeds (Syagrus romanzoffiana [Cham.] Glassman) Tratamiento de pre-despulpado y despulpado sobre la emergencia de semillas de palma reina (Syagrus romanzoffiana [Cham.] Glassman) LUCAS MARQUEZAN NASCIMENTO1 EDUARDO PRADI VENDRUSCOLO2, 4 LUIZ FERNANDES CARDOSO CAMPOS1 LISMAÍRA GONÇALVES CAIXETA GARCIA1 LARISSA LEANDRO PIRES1 ALEXANDER SELEGUINI3 Syagrus romanzoffiana under conditions of Brazilian Cerrado. Photo: L.M. Nascimento ABSTRACT The propagation of the palm Syagrus romanzoffiano is done sexually with seeds, making the process of obtai- ning new plants slow and difficult, especially on large scales. In addition, seed germination is slow, uneven and susceptible to degradation and loss of vigor because of embryo deterioration, even under laboratory conditions. As a result of the lack of information on efficient depulping methods for queen palm fruits, the present study aimed to establish a depulping methodology that is less aggressive to embryos, maintaining emergence quality. This experiment was carried out in Goiânia, Brazil, using fruits from eight stock plants submitted to three pre-depulping treatments (control, fermentation and drying) and two depulping me- thods (industrial depulping and concrete-mixer with the addition of gravel). After the different pre-sowing processes, the fresh and dry pyrenes mass, remaining fibers adhered to the pyrene and seedling emergence were evaluated. The pulper removed an average of 45% more pyrene pulp than the concrete mixer. However, these methodologies did not result in differences in the emergence of plants, which was affected only by the pre-depulping treatment, with superiority in the use of fresh fruits. -

Análisis Aeropalinológico Del Parque Nacional El Palmar
Bol. Soc. Argent. Bot. 52 (3) 2017 N. E. Muñoz et al. - Análisis aeropalinológico del Parque NacionalISSN El0373-580 Palmar X Bol. Soc. Argent. Bot. 52 (3): 473-496. 2017 ANÁLISIS AEROPALINOLÓGICO EN TRES ÁREAS DE VEGETACIÓN DENTRO DEL PARQUE NACIONAL EL PALMAR (COLÓN, ENTRE RÍOS) Y SU RELACIÓN CON LA VEGETACIÓN LOCAL Y REGIONAL NADIA E. MUÑOZ1, MERCEDES DI PASQUO1, FERNANDO BIGANZOLI2 y WILLIAM B. BATISTA2,3 Summary: Aeropalinological analysis in three vegetation areas within El Palmar National Park (Colón, Entre Ríos) and its relationship with the local and regional vegetation. The diversity of pollen rain monthly collected during two years (2011-2013) from the atmosphere in Tauber traps located at three sites in El Palmar National Park (Entre Ríos Province) is used to characterize the source vegetation. Site 1 is a mixed area composed of grassland, palm savanna, and wetland communities, site 2 is a grassland area and site 3 is a dense palm savanna. A total of 71 pollen-grain types grouped in 43 families coming from local, regional and extra- regional areas are identified. Of them, sixteen pollen types with more than 1% of Annual Pollen Influx in at least two samples were used in this analysis. Different factors involved in quali-quantitave changes of taxa during the observation interval (e.g. pollination affinity, origin of pollen grains, canopy effect, meteorological variables) are further considered. The floral composition of each site compared to their palynoassemblages revealed that site 2 is characterized by a high abundance of Asteraeceae-Asteroideae, with an increase in the value of Vernonia (Asteraceae Cichoroidea) and Lamiaceae during the second year. -
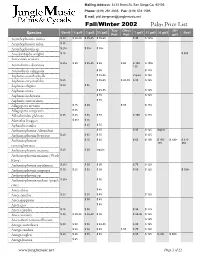
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Upper Paraná Atlantic Forest Program
Upper Paraná Atlantic Forest Program The Upper Paraná Atlantic Forest – locally called Selva Misionera or Paranaense - is one of the world’s most biodiverse subtropical ecoregions. Spread across three countries – Argentina, Brazil and Paraguay – these forests are one of the planet’s most threatened areas as a result of past and present deforestation. Over the last decade, Fundación Vida Silvestre Argentina (FVSA), WWF’s associate organization, has been working towards the conservation of the forests of the Misiones Province. These forests –located in northeastern Argentina- are one of the largest forest blocks that still remains, and one of the few that still houses a jaguar population. From the heart of the jungle, FVSA-WWF’s office in Puerto Iguazú, just a few kilometers away from the impressive scenario offered by the Iguazú Falls, promotes conservation and sustainable development actions. Some of these undertakings are the Misiones Green Corridor and the Biodiversity Vision, a planning tool for the ecoregion. The Upper Paraná Atlantic Forest (UPAF) covers The Misiones Green Corridor was singled out in most part of the Misiones Province in northeastern the Biodiversity Vision as the most important area Argentina, and expands through the south of where to focus local actions with regional impact. Brazil and eastern Paraguay. Due to its Within this framework, FVSA-WWF continues importance, it was included in the WWF “Global pressuring for the complete implementation of the 200” list as one of the most endangered ecoregions Green Corridor Law, which created a 1,1 million in the world. hectare Integral Conservation and Sustainable Development Area. -

Redalyc.ECOLOGICAL STRATEGIES and IMPACT of WILD BOAR IN
Mastozoología Neotropical ISSN: 0327-9383 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Cuevas, M. Fernanda; Ojeda, Ricardo A.; Jaksic, Fabian M. ECOLOGICAL STRATEGIES AND IMPACT OF WILD BOAR IN PHYTOGEOGRAPHIC PROVINCES OF ARGENTINA WITH EMPHASIS ON ARIDLANDS Mastozoología Neotropical, vol. 23, núm. 2, 2016, pp. 239-254 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Available in: http://www.redalyc.org/articulo.oa?id=45750282004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Mastozoología Neotropical, 23:239-254, Mendoza, 2016 Copyright ©SAREM, 2016 http://www.sarem.org.ar Versión impresa ISSN 0327-9383 http://www.sbmz.com.br Versión on-line ISSN 1666-0536 Sección Especial MAMÍFEROS EXÓTICOS INVASORES ECOLOGICAL STRATEGIES AND IMPACT OF WILD BOAR IN PHYTOGEOGRAPHIC PROVINCES OF ARGENTINA WITH EMPHASIS ON ARIDLANDS M. Fernanda Cuevas1, Ricardo A. Ojeda1 and Fabian M. Jaksic2 1 Grupo de Investigaciones de la Biodiversidad (GiB), IADIZA, CCT Mendoza CONICET, CC 507, 5500 Mendoza, Argentina. [Correspondence: <[email protected]>]. 2 Centro de Ecología Aplicada y Sustentabilidad (CAPES), Pontificia Universidad Católica de Chile, Casilla 114-D, Santiago, Chile ABSTRACT. Wild boar is an invasive species introduced to Argentina for sport hunting purposes. Here, this spe- cies is present in at least 8 phytogeographic provinces but we only have information in four of them (Pampean grassland, Espinal, Subantarctic and Monte Desert). We review the ecological strategies and impact of wild boar on ecosystem processes in these different phytogeographic provinces and identify knowledge gaps and research priorities for a better understanding of this invasive species in Argentina. -

Quality of Life in Argentina
Belgeo Revue belge de géographie 4 | 2013 Miscellaneous Quality of life in Argentina: The environmental dimension at a departmental scale La qualité de vie en Argentine : dimension environnementale à l’échelle des départements Guillermo Ángel Velázquez et Juan Pablo Celemín Édition électronique URL : http://journals.openedition.org/belgeo/11794 DOI : 10.4000/belgeo.11794 ISSN : 2294-9135 Éditeur : National Committee of Geography of Belgium, Société Royale Belge de Géographie Édition imprimée Date de publication : 31 décembre 2013 ISSN : 1377-2368 Référence électronique Guillermo Ángel Velázquez et Juan Pablo Celemín, « Quality of life in Argentina: The environmental dimension at a departmental scale », Belgeo [En ligne], 4 | 2013, mis en ligne le 30 juin 2014, consulté le 30 avril 2019. URL : http://journals.openedition.org/belgeo/11794 ; DOI : 10.4000/belgeo.11794 Ce document a été généré automatiquement le 30 avril 2019. Belgeo est mis à disposition selon les termes de la licence Creative Commons Attribution 4.0 International. Quality of life in Argentina: The environmental dimension at a departmental s... 1 Quality of life in Argentina: The environmental dimension at a departmental scale La qualité de vie en Argentine : dimension environnementale à l’échelle des départements Guillermo Ángel Velázquez et Juan Pablo Celemín Introduction 1 The analysis of the Quality of Life from a geographic perspective relies mainly on the development of indices with the highest possible level of territorial disaggregation and reflecting the relative wellbeing of the population. Earlier indices developed for Argentina (Velázquez, 2008; 2010a) provided basically two dimensions: a) socio-economic and b) environmental. Socio-economic dimension embraces such indicators as education, health and housing, while environmental one considers three aspects: nature-based recreational resources, socially constructed recreational resources and environmental problems. -

Sfps Fall 2011 Sale Plant List
SFPS FALL 2011 SALE PLANT LIST PLANTS VENDOR # Palms Acanthophoenix rubra 35 Acoelorrhaphe wrightii 26, 67 Acrocomia aculeata 50, 67 Actinokentia divaricata 35, 57, 66, 68, 72 Actinorhytis calapparia 72 Adonidia merrillii 31, 57, 66, 89 Adonidia merrillii var. "Golden Form" 35 Aiphanes aculeata = Aiphanes horrida - Aiphanes caryotifolia = Aiphanes horrida - Aiphanes erosa = Aiphanes minima - Aiphanes horrida 35, 68, 72 Aiphanes minima 68 Aiphanes vincentiana = Aiphanes minima - Allagoptera arenaria 57, 66, 67, 68, 72 Allagoptera campestris 67 Allagoptera leucocalyx 57 Alloschmidia glabrata = Basselinia glabrata - Alsmithia longipes = Heterospathe longipes - Archontophoenix cunninghamiana var. 'Illawara' 68 Archontophoenix maxima 67, 72 Archontophoenix myolensis 50, 66, 67, 68 Archontophoenix purpurea 57, 66, 72 Archontophoenix tuckeri 66, 68 Areca aliceae = Areca triandra - Areca camarinensis 57, 68 Areca catechu 57, 67, 72 Areca catechu var. 'Dwarf' 35, 50 Areca hutchinsoniana 68 Areca ipot 67 Areca latiloba = Areca montana - Areca macrocalyx var. 'Red Form' 35, 57, 68 Areca macrocarpa 68 Areca montana 57 Areca triandra 68, 72 Areca vestiaria 25, 35, 57, 67, 68 Areca vestiaria var. 'Orange Form' 25, 57, 67, 72 Areca vestiaria var. 'Maroon Leaf' 35, 57, 67 Areca vestiaria var. 'Red Leaf' 57, 67, 72 Areca sp. 'Yellow Crownshaft' 25 Arenga ambong = Arenga undulatifolia - Arenga brevipes 57 Arenga caudata 66 Arenga engleri 31, 66, 68, 72 Arenga hookeriana 35, 57, 66, 72 Arenga microcarpa 26, 66 Arenga obtusifolia 57, 66 PLANTS VENDOR # Arenga pinnata 50, 57, 66, 67, 68 Arenga porphyrocarpa 66 Arenga tremula 26, 57, 66, 68, 72 Arenga undulatifolia 35, 57, 66, 67 Arenga westerhoutii 68 Asterogyne martiana 57, 68, 72 Astrocaryum acaule 72 Astrocaryum alatum 35, 50, 57, 67 Astrocaryum mexicanum 72 Astrocaryum murumuru 72 Attalea butyracea 57, 67, 72 Attalea cohune 35 Attalea phalerata 50, 91 Attalea rostrata 68 Attalea speciosa 50, 66 Bactris bidentula 72 Bactris gasipaes 67 Bactris gasipaes var. -

Four New Taxa of Acaulescent Syagrus (Arecaceae) from Brazil
Phytotaxa 188 (1): 001–013 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.188.1.1 Four new taxa of acaulescent Syagrus (Arecaceae) from Brazil LARRY R. NOBLICK1, HARRI LORENZI2 & VINICIUS C. SOUZA3 1 Montgomery Botanical Center, Miami, FL, U.S.A.; email: [email protected]; 2Jardim Plantarum, Nova Odessa, SP, Brazil, email: [email protected]; 3Departamento de Ciência Biológicas, ESALQ-USP, Piricicaba, SP, Brazil; email: [email protected] Abstract Three new species and a new subspecies of acaulescent Syagrus palms are described as new to science. These occur in the central western cerrado region of Brazil: Syagrus emasensis and S. menzeliana from southwestern Goiás, S. guimaraesensis from south central Mato Grosso and finally S. graminifolia subsp. cabraliensis from north central Minas Gerais. Keywords: Arecales, Arecoideae, Cocoseae, Palmae Introduction The genus Syagrus currently contains 56 species (Lorenzi et al. 2010, Noblick 2014, Soares et al. 2013). Of these 56 species, 26 are acaulescent (Noblick 2013). The word acaulescent translates as “without a trunk” and a trunk is defined as an above ground stem. While these palms appear to have no above ground stem, they do all have short, subterranean stems. Many of these acaulescent Syagrus are difficult to identify from herbarium material, but leaflet anatomy has been found to be useful in their identification (Glassman 1972, Noblick 2013). The discovery that the acaulescent Syagrus petraea (Mart.) Beccari (1916: 467) was a Bolivian endemic (Noblick et al. 2010), rather than the broadly distributed morphologically variable species that many botanists assumed that it was (Henderson et al. -

Cyanocharax Alburnus (Hensel, 1870) (Characiformes: Characidae): First Distribution Record in Argentina Istributio D Lucila C
Check List 8(3): 581-583, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution N Cyanocharax alburnus (Hensel, 1870) (Characiformes: Characidae): First distribution record in Argentina ISTRIBUTIO D Lucila C. Protogino 1,2* and Amalia M. Miquelarena 1,2 RAPHIC G EO 1 Instituto de Limnología “Dr. Raúl A. Ringuelet” (CONICET CCT La Plata-UNLP). CC 712, 1900. La Plata, BA, Argentina. G 2 Museo de La Plata, División Zoología Vertebrados. Paseo del Bosque s/n, 1900. La Plata, BA, Argentina. N * Corresponding author. E-mail: [email protected] O OTES N Abstract: This note reports the presence of Cyanocharax alburnus in the Argentinian Mesopotamia. This represents the southern distributional limit for the species in South America and the first record for Argentina’s fresh water fish fauna. The genus Cyanocharax Malabarba and Weitzman, 2003, included in the family Characidae, comprises small- sized species: C. alburnus (Hensel, 1870), C. alegretensis Villaguay creek is a relatively extensive watercourse, with Malabarba and Weitzman, 2003; C. dicropotamicus thesome bottom sectors consists about 50of mudm wide and and clay. pools Entre over Ríos 2 province m deep. Malabarba and Weitzman, 2003; C. itaimbe Malabarba and The banks are vegetated with grasses and other plants, and Weitzman, 2003; C. lepiclastus Malabarba, Weitzman and one of the areas of highest biodiversity in Argentina (López Casciotta, 2003; C. tipiaia Malabarba and Weitzman, 2003 etrepresents, al along with the rest of the Mesopotamic region, and C. uruguayensis Atlantic drainage basins in southeastern Brazil, in the states . -

Uso Prehispánico De Las Palmeras Syagrus Romanzoffiana Y Butia
Uso prehispánico de las palmeras Syagrus romanzoffiana y Butia yatay en el Nordeste argentino: aportes desde la etnografía y la biometría RMA Mariano Bonomo* y Luis Enrique Capeletti** Dossier *CONICET-División Arqueología del Museo de La Plata, Facultad de Ciencias Arqueología del Litoral Naturales y Museo, Universidad Nacional de La Plata, Argentina. [email protected]; **Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Argentina. [email protected] Resumen La distribución meridional de las palmeras de la familia Arecaceae (=Palmae) en Sudamérica alcanza el sur de la cuenca del Plata. Este artículo se centra en dos especies de esta familia, Syagrus romanzoffiana y Butia yatay, cuyos micro y macrorrestos se han preservado en los sitios arqueológicos del Holoceno tardío en el Nordeste argentino. El registro arqueológico indica que fueron importantes para la alimentación y, seguramente, en la tecnología. Además, grupos etnográficos pertenecientes a distintos troncos lingüísticos (tupí, macro-jê, guaycurú) muestran que estos vegetales tuvieron un rol central en el Plata. A pesar de ello, hasta el presente estudio no se había efectuado en el Nordeste un análisis sistemático y desde distintas líneas de trabajo que permitan incrementar la información taxonómica y económica que se puede obtener de los restos de ambas palmeras. A partir de la revisión de la literatura etnográfica, la determinación de la utilidad neta de los frutos y el estudio de los atributos cuali-cuantitativos de los endocarpos, se establecen los usos potenciales de este recurso y la productividad de sus frutos y se brindan herramientas para la identificación taxonómica. Todo ello permite profundizar los conocimientos sobre la utilización prehispánicas de las palmeras en la región. -

PHENOLOGY, BIOMETRICS and FRUITS PRODUCTION of Attalea
Facultad de Ciencias ACTA BIOLÓGICA COLOMBIANA Departamento de Biología http://www.revistas.unal.edu.co/index.php/actabiol Sede Bogotá ARTÍCULO DE INVESTIGACIÓN / RESEARCH ARTICLE BOTÁNICA PHENOLOGY, BIOMETRICS AND FRUITS PRODUCTION OF Attalea nucifera (ARECACEAE) IN COLOMBIA FENOLOGÍA, PARÁMETROS BIOMÉTRICOS Y PRODUCTIVIDAD DE FRUTOS DE Attalea nucifera (ARECACEAE) EN COLOMBIA Ivón Jiménez-Morera1 , Néstor García1 1Departamento de Biología, Facultad de Ciencias, Pontificia Universidad Javeriana, Carrera 7 No. 40 – 62, Bogotá, Colombia *For correspondence: [email protected] Received: 06th February 2019, Returned for revision: 03rd May 2019, Accepted: 28th May 2019. Associate Editor: Xavier Marquínez-Casas. Citation/Citar este artículo como: Jiménez-Morera I, García N. Phenology, biometrics and fruits production of Attalea nucifera (Arecaceae) in Colombia. Acta biol. Colomb. 2020;25(1):104-111. DOI: http://dx.doi.org/10.15446/abc.v25n1.77701 ABSTRACT Attalea nucifera is a threatened palm endemic to the Magdalena River basin in Colombia. In the past its seeds were consumed by the inhabitants of the town of Guaduas, Cundinamarca, although currently its use is less frequent. To assess the productive potential of this palm, we studied its phenology, biometric parameters, and fruit productivity in a forest relict in Guaduas. Field work was carried out between April 2016 and March 2017. The reproductive cycle of this species lasted approximately 12 and a half months from bud to fruit ripening. Although bud production occurred throughout the year, it increased during periods of greatest rainfall. Flowering peaks occurred towards the end of the rainy season and fruits ripened towards the period of low rainfall. We found a positive correlation between the number of leaves in the crown and the production of reproductive structures (rs = 0.447, p = 0.004). -

From Barcelona to Bordighera: Palm Gardens on Mediterranean Shores
PALMS Pintaud: Mediterranean Palm Gardens Volume 46(3) 2002 From Barcelona JEAN-CHRISTOPHE PINTAUD to Bordighera: IRD, UMR DGPC Laboratoire GENETROP 911 Avenue Agropolis Palm Gardens on BP 64501 34394 Montpellier Mediterranean Cedex 5, France Shores 1. Native Chamaerops humilis south of Barcelona. The species occurs in large numbers on rocky limestone slopes in front of the sea. Palms and palm landscapes are acknowledged as symbols of exoticism and as such contribute greatly in attracting people, especially tourists, to the Mediterranean region. Thus there is a need to preserve the region’s many historical palm gardens. Municipalities play a very important role in the conservation of the palm heritage. Nice has a network of parks, mostly originating from ancient private properties, and is developing a new botanical garden. The cities of San Remo, Menton, Cannes, Hyères, Toulon and Le Pradet are developing comprehensive new palm collections in old, renovated gardens, often in association with the French Palm Society (Fous de Palmiers). PALMS 46(3): 149–153 149 PALMS Pintaud: Mediterranean Palm Gardens Volume 46(3) 2002 The northwestern part of the Mediterranean Sea cultivation, the date palm will completely and adjacent European continent have an disappear. unusually warm climate for this latitude Bordighera’s date palms also played an important (41–44°N). Palms reach the northern limit of their role in several respects in the 19th century natural distribution there, with the Mediterranean development of the Riviera. Early palm land- fan palm Chamaerops humilis. This species is a scaping was made with Bordighera’s palms, the typical component – an indicator – of the warmest only significant source of well-grown plants before Mediterranean vegetation zone.