COMPARATIVE EXPERIMENTAL TAPHONOMY of EIGHT MARINE ARTHROPODS INDICATES DISTINCT DIFFERENCES in PRESERVATION POTENTIAL by ADIEL€ A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Learning in Stomatopod Crustaceans
International Journal of Comparative Psychology, 2006, 19 , 297-317. Copyright 2006 by the International Society for Comparative Psychology Learning in Stomatopod Crustaceans Thomas W. Cronin University of Maryland Baltimore County, U.S.A. Roy L. Caldwell University of California, Berkeley, U.S.A. Justin Marshall University of Queensland, Australia The stomatopod crustaceans, or mantis shrimps, are marine predators that stalk or ambush prey and that have complex intraspecific communication behavior. Their active lifestyles, means of predation, and intricate displays all require unusual flexibility in interacting with the world around them, imply- ing a well-developed ability to learn. Stomatopods have highly evolved sensory systems, including some of the most specialized visual systems known for any animal group. Some species have been demonstrated to learn how to recognize and use novel, artificial burrows, while others are known to learn how to identify novel prey species and handle them for effective predation. Stomatopods learn the identities of individual competitors and mates, using both chemical and visual cues. Furthermore, stomatopods can be trained for psychophysical examination of their sensory abilities, including dem- onstration of color and polarization vision. These flexible and intelligent invertebrates continue to be attractive subjects for basic research on learning in animals with relatively simple nervous systems. Among the most captivating of all arthropods are the stomatopod crusta- ceans, or mantis shrimps. These marine creatures, unfamiliar to most biologists, are abundant members of shallow marine ecosystems, where they are often the dominant invertebrate predators. Their common name refers to their method of capturing prey using a folded, anterior raptorial appendage that looks superficially like the foreleg of a praying mantis. -
Tropical Hermit Crab Clibanarius Clibanarius
MARINE ECOLOGY - PROGRESS SERIES Vol. 8: 197-201, 1982 Published May P Mar. Ecol. Prog. Ser. / Reproduction of the Continuously Breeding Tropical Hermit Crab Clibanarius clibanarius Sudha Varadarajan* and T. Subramoniam Department of Zoology, University of Madras, Madras 600005,India ABSTRACT: On the Indian east coast, the hermit crab Clibanarius clibanarius breeds continuously with peak activity from September to January, corresponding to the onset of the retreating monsoon. Individuals within the population breed asynchronously and vary widely in their carapace lengths. probably because of steady, year-round recruitment. Our data on reproduction emphasize the flexible use of an apparently stable, tropical environment by these crabs. INTRODUCTION 18 m. In January and February, 1976 and January, 1977 all crabs were scarce and few could be collected. In tropical marine waters, where sea temperatures Females, smaller in size than males, often occupied fluctuate little seasonally, many invertebrates are smaller gastropod shells of different species of Murex, known to reproduce throughout the year (Giese and Bursa, Babylonia or Turitella. After removal from the Pearse, 1974). In Indian waters a major factor that shell, the length from the tip of the rostrum to the influences intertidal as well as offshore forms is the posterior indentation on the mid-dorsal line of the monsoon rain that differs in time and intensity on the cephalothorax was taken as carapace length. The soft east and west coasts (Panikkar and Jayaraman, 1966). abdomen was opened, the ovaries were separated from Semiannual breeding patterns have been reported for the hepatic tissue and gonad-indexes and hepatic a number of species on the east coast of India (Giese indexes (Giese, 1967) and egg mass indexes (Sub- and Pearse, 1974), where little rain falls during the ramoniam, 1979) were calculated' '. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
Lobsters LOBSTERS§
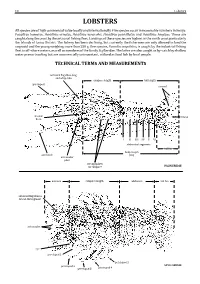
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Decapode.Pdf
We are pleased and honored to welcome at the Paléospace Museum of Villers-sur-Mer the “6th Symposium on Mesozoic and Cenozoic Decapod Crustaceans”. Villers-sur-Mer is a place universally known by specialists and amateurs of palaeontology due to its famous Vaches Noires cliffs. Villers-sur-Mer has also the distinction of being the only French seaside resort located on the Greenwich Meridian line. The Paléospace is a Museum funded in 2011 with the label Musée de France. Three main animations linked to the Time are presented: palaeontology, astronomy and nature with the neighbouring marsh. The museum is in a constant evolution. For instance, an exhibition specially dedicated to dinosaurs was opened two years ago and a planetarium will open next summer. Every year a very high quality temporary exhibition takes place during the summer period with very numerous animations during all the year. The Paléospace does not stop progressing in term of visitors (56 868 in 2015) and its notoriety is universally recognized both by the other museums as by the scientific community. We are very proud of these unexpected results. We thank the dynamism and the professionalism of the Paléospace team which is at the origin of this very great success. We wish you a very good stay at Villers-sur-Mer, a beautiful visit of the Paléospace and especially an excellent congress. Jean-Paul Durand, Mayor and President of Paléospace MOT DU MAIRE DE VILLERS-SUR-MER Nous sommes très heureux et très honorés d’accueillir à Villers-sur-Mer, le « 6e Symposium on Mesozoic and Cenozoic Decapod Crustaceans » dans le cadre du Paléospace. -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

Pagurid Crabs (Decapoda Anomura) from St
PAGURID CRABS (DECAPODA ANOMURA) FROM ST. JOHN, VIRGIN ISLANDS, WITH DESCRIPTIONS OF THREE NEW SPECIES 1) BY ANTHONY J. PROVENZANO, Jr. Institute of Marine Science,University of Miami, Florida, U.S.A. INTRODUCTION As part of a survey of the marine fauna and flora of the recently established U. S. Virgin Islands National Park at St. John, a series of collections have been made by Marine Laboratory personnel. Among the Crustacea taken were a number of new records for the Virgin Islands, including three species not referable to any yet named. The present report deals with the hermit crabs of the families Diogenidae and Paguridae; these families corresponding to those of MacDonald, Pike & Williamson ( 1 9 5 7 ) . Synonymies are restricted to the original description and one or more references usually containing more complete information. Size of specimens where given refers to carapace length. The station numbers refer to records kept by Herman Kumpf while at St. John; the field data being on file in the Marine Laboratory Museum. Unless otherwise noted, the general locality for material examined is St. John. Holotypes are deposited in the U. S. National Museum, while most of the remaining material is deposited in the University of Miami Marine Laboratory Museum (UMML). The writer is indebted to Dr. Marvin L. Wass of the Virginia Fisheries Labora- tory for examining the three new species and for comments incorporated in the manuscript. He would also like to thank Dr. John Randall and his co-workers who made special efforts to collect hermit crabs during their general field studies. -

A New Early Miocene Barnacle Lineage and the Roots of Sea-Turtle Fouling Chelonibiidae (Cirripedia, Balanomorpha) Mathias Harzhausera∗, William A
Journal of Systematic Palaeontology, Vol. 9, Issue 4, December 2011, 473–480 A new Early Miocene barnacle lineage and the roots of sea-turtle fouling Chelonibiidae (Cirripedia, Balanomorpha) Mathias Harzhausera∗, William A. Newmanb and Patrick Grunertc aGeological-Paleontological Department, Natural History Museum Vienna, Burgring 7, A-1014 Vienna, Austria; bScripps Institution of Oceanography, UCSD, 9500 Gilman Drive, La Jolla CA 92093, USA; cInstitute for Earth Sciences, University of Graz, Heinrichstraße 26, A-8010 Graz, Austria (Received 6 May 2010; accepted 15 Jul 2010; printed 29 November 2011) The origin of the mainly sea-turtle fouling balanomorph family Chelonibiidae is still poorly documented. Aside from an Eocene erratic specimen assigned to an extinct subfamily, the extant subfamily Chelonibiinae did not appear in the fossil record before the Late Miocene. Protochelonibiinae Harzhauser & Newman subfam. nov. is here introduced as an extinct sister-group of Chelonibiinae. The subfamily is known so far only from the proto-Mediterranean and the Paratethys seas and ranged from Early Miocene to Late Pliocene. Members of the subfamily are characterized by large walls with tripartite rostra which display distinct sutures on the external surface. The tripartite rostrum, however, has evolved independently several times in the evolution of the balanomorphs and cannot be treated as synapomorphy. The subfamily comprises one new genus and two species. Protochelonibia Harzhauser & Newman gen. nov. is the type genus of Protochelonibiinae and Protochelonibia submersa Harzhauser & Newman sp. nov. is introduced as type species of this genus. Chelonobia Capellinii [sic] De Alessandri, 1895, from the Late Pliocene of Italy, reassigned as Protochelonibia capellinii (De Alessandri, 1895), is the youngest record of the subfamily. -

Cannibalism and Habitat Selection of Cultured Chinese Mitten Crab: Effects of Submerged Aquatic Vegetation with Different Nutritional and Refuge Values
water Article Cannibalism and Habitat Selection of Cultured Chinese Mitten Crab: Effects of Submerged Aquatic Vegetation with Different Nutritional and Refuge Values Qingfei Zeng 1,*, Erik Jeppesen 2,3, Xiaohong Gu 1,*, Zhigang Mao 1 and Huihui Chen 1 1 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China; [email protected] (Z.M.); [email protected] (H.C.) 2 Department of Bioscience, Aarhus University, Vejlsøvej 25, 8600 Silkeborg, Denmark; [email protected] 3 Sino-Danish Centre for Education and Research, University of CAS, Beijing 100190, China * Correspondence: [email protected] (Q.Z.); [email protected] (X.G.) Received: 26 September 2018; Accepted: 26 October 2018; Published: 29 October 2018 Abstract: We examined the food preference of Chinese mitten crabs, Eriocheir sinensis (H. Milne Edwards, 1853), under food shortage, habitat choice in the presence of predators, and cannibalistic behavior by comparing their response to the popular culture plant Elodea nuttallii and the structurally more complex Myriophyllum verticillatum L. in a series of mesocosm experiments. Mitten crabs were found to consume and thus reduce the biomass of Elodea, whereas no negative impact on Myriophyllum biomass was recorded. In the absence of adult crabs, juveniles preferred to settle in Elodea habitats (appearance frequency among the plants: 64.2 ± 5.9%) but selected for Myriophyllum instead when adult crabs were present (appearance frequency among the plants: 59.5 ± 4.9%). The mortality rate of mitten crabs in the absence of plant shelter was higher under food shortage, primarily due to cannibalism. -

Brachyura, Panopeidae)
Invertebrate Reproduction & Development ISSN: 0792-4259 (Print) 2157-0272 (Online) Journal homepage: https://www.tandfonline.com/loi/tinv20 Relative growth, morphological sexual maturity, heterochely, and handedness in Panopeus occidentalis (Brachyura, Panopeidae) Fernanda Monchelato Santos, Régis Augusto Pescinelli, João Alberto Farinelli Pantaleão & Rogério Caetano Costa To cite this article: Fernanda Monchelato Santos, Régis Augusto Pescinelli, João Alberto Farinelli Pantaleão & Rogério Caetano Costa (2018) Relative growth, morphological sexual maturity, heterochely, and handedness in Panopeusoccidentalis (Brachyura, Panopeidae), Invertebrate Reproduction & Development, 62:2, 74-81, DOI: 10.1080/07924259.2017.1415987 To link to this article: https://doi.org/10.1080/07924259.2017.1415987 Published online: 15 Dec 2017. Submit your article to this journal Article views: 76 View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tinv20 INVERTEBRATE REPRODUCTION & DEVELOPMENT, 2018 VOL. 62, NO. 2, 74–81 https://doi.org/10.1080/07924259.2017.1415987 Relative growth, morphological sexual maturity, heterochely, and handedness in Panopeus occidentalis (Brachyura, Panopeidae) Fernanda Monchelato Santosa, Régis Augusto Pescinellia , João Alberto Farinelli Pantaleãoa,b and Rogério Caetano Costaa aLaboratory of Biology of Marine and Freshwater Shrimp (LABCAM), Faculty of Sciences, Department of Biological Sciences, São Paulo State University (UNESP), São Paulo,