(Teleostei: Gadiformes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Teleostei: Gadiformes: Moridae), Antimora Microlepis and Physiculus Japonicus, from the Western North Pacific
Species Diversity, 2007, 12, 17–27 Pelagic Juveniles of Two Morids (Teleostei: Gadiformes: Moridae), Antimora microlepis and Physiculus japonicus, from the Western North Pacific Makoto Okamoto1, Naoshi Sato2, Takashi Asahida2 and Yoshiro Watanabe3 1 Tohoku National Fisheries Research Institute, 3-27-5 Shinhama-cho, Shiogama, Miyagi, 985-0001 Japan E-mail: [email protected] 2 School of Fisheries Sciences, Kitasato University, 160-4 Okirai, Sanriku-cho, Ofunato, Iwate, 022-0101 Japan 3 Ocean Research Institute, University of Tokyo, Minamidai, Nakano-ku, Tokyo, 164-8639 Japan (Received 1 July 2006; Accepted 20 November 2006) Pelagic juveniles of two morids, Antimora microlepis Bean, 1890 and Physiculus japonicus Hilgendorf, 1879, were collected by midwater trawl (0–20 m depth) from transition waters between the Oyashio and Kuroshio fronts of the western North Pacific in May, 1989. Juveniles of A. microlepis (13 specimens, 30.2–54.3 mm standard length, SL) characteristically have an elongated body, posteriorly positioned anus, 24–25 precaudal vertebrae, chin with a barbel, no ventral luminous organ, elongated pelvic fin rays, and a non-protruding snout. Juveniles of P. japonicus (six specimens, 20.4–39.0 mm SL) characteristically have an elongated body, chin with a barbel, ventral lu- minous organ anterior to the anus, elongated pelvic fin rays, a pointed cau- dal fin, 9–10 dorsal fin rays, 66–70 second dorsal fin rays, 68–72 anal fin rays, six pelvic fin rays, 16ϩ41–42 vertebrae, and ca. 130 longitudinal scales. Key Words: Teleostei, Gadiformes, Moridae, Antimora microlepis, Physicu- lus japonicus, pelagic juvenile. Introduction The family Moridae currently comprises 18 genera and about 110 species, which occur widely from coastal to pelagic waters over the entire world except in the Arctic Ocean (Paulin 1989a; Okamura 1995; Eschmeyer 1998). -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -
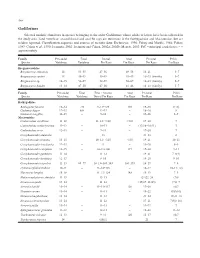
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Determining the Diet of New Zealand King Shag Using DNA Metabarcoding
Determining the diet of New Zealand king shag using DNA metabarcoding New Zealand King Shag, (Leucocarbo carunculatus) on Blumine Island, Marlborough Sounds, New Zealand in 2016 (Wikipedia commons). Aimee van der Reis & Andrew Jeffs Report Prepared For: Department of Conservation, Conservation Services Programme, Project BCBC2019-05. DOC MarineDRAFT Science Advisors Graeme Taylor and Dr Karen Middlemiss. November 2020 Reports from Auckland UniServices Limited should only be used for the purposes for which they were commissioned. If it is proposed to use a report prepared by Auckland UniServices Limited for a different purpose or in a different context from that intended at the time of commissioning the work, then UniServices should be consulted to verify whether the report is being correctly interpreted. In particular it is requested that, where quoted, conclusions given in Auckland UniServices reports should be stated in full. INTRODUCTION The New Zealand king shag (Leucocarbo carunculatus) is an endemic seabird that is classed as nationally endangered (Miskelly et al., 2008). The population is confined to a small number of colonies located around the coastal margins of the outer Marlborough Sounds (South Island, New Zealand); with surveys suggesting the population is currently stable (~800 individuals surveyed in 2020; Aquaculture New Zealand, 2020; Schuckard et al., 2015). Monitoring the colonies has become a priority and research is being conducted to better understand their population dynamics and basic ecology to improve the management of the population, particularly in relation to human activities such as fishing, aquaculture and land use (Fisher & Boren, 2012). The diet of the New Zealand king shag is strongly linked to the waters surrounding their colonies and it has been suggested that anthropogenic activities, such as marine farm structures, may displace foraging habitat that could affect the population of New Zealand king shag (Fisher & Boren, 2012). -

MOR 1983 FAO SPECIES IDENTIFICATION SHEETS FISHING AREA 51 (W. Indian Ocean) MORIDAE Moras Body Relatively Elongate, Tapering To
click for previous page MOR 1983 FAO SPECIES IDENTIFICATION SHEETS FISHING AREA 51 (W. Indian Ocean) MORIDAE Moras Body relatively elongate, tapering to a narrow caudal peduncle. Mouth terminal or inferior; teeth few or lacking on head of vomer (roof of mouth). No spines in fins; 2 or 3 dorsal fins and 1 or 2 anal fins; pelvic fins thoracic, never very close together; caudal fin always separate from dorsal and anal fins. Small cycloid scales on body and head. Anterior paired projections of swimbladder attached to a membranous area at the rear of the cranium. Colour: variable, black or grey to light brown or pink; some species may have iridescent areas. Fishes of the continental slope and abyssal depths. Locally abundant in some habitats but not commercial in the area. Taken in bottom trawls. Lepidion Physiculus Tripterophycis - 2 - FAO Sheets MORIDAE Fishing Area 51 SIMILAR FAMILIES OCCURRING IN THE AREA: Macrouridae: no caudal fin. Ophidiidae and Bythitidae: ventral fins close Macrouridae together, dorsal,caudal and anal fins joined in most. Ateleopodidae: no scales; no free caudal fin; a single dorsal fin far forward on the body. Ophidiidae Ateleopodidae pelvic fins Moridae Ophidiidae Underside of head KEY TO GENERA OCCURRING IN THE AREA: 1a. Mouth inferior, beneath a prominent, flattened, pointed, bony snout (Fig.1) ................................ Antimora 1b. Mouth terminal to slightly inferior; snout nor- mal Antimora Fig.1 2a. One or more small, dark, scaleless patches (covering a light organ) on the belly (Fig.2) patch covering light organ pelvic fin anus Fig.2 - 3 - FAO Sheets MORIDAE Fishing Area 51 3a. -

346 2.8 Family Moridae
click for previous page 346 Geographical Distribution : Central western Atlantic (Fig. 748). Habitat and Biology : Outer shelf and upper slope, on soft bottoms. Size : Maximum total length recorded from Suriname, 27.7 cm. Interest to Fisheries : None at present. Often taken in large quantities at depths of 400 to 500 m in the northern Gulf of Mexico. Literature : Goode & Bean (1896) Fig. 748 2.8 FAMILY MORIDAE MOR Family Name with Reference : Morini Moreau, 1881, Hist.nat.Poiss.France, 3:247. FAO Names : En - Moras; Fr - Moros; Sp - Moros General Features : Body, in most species, tapering to a very narrow caudal peduncle. No V-shaped ridge on top of skull; gill openings wide; extending upward above level of pectoral fins; mouth usually terminal or inferior. Teeth few or lacking on head of vomer (roof of mouth). Fins lacking spines; two or three dorsal fins and one or two anal fins; pelvic fins thoracic, never very close together at their bases; caudal fin always separate from dorsal and anal fins, end externally symmetrical. Scales overlapping and rounded, not set at right angles to each other. Spine on top of first vertebra tightly connected to a narrow crest at rear of skull. Anterior paired projections of swimbladder attached to a membranous area at the rear of the cranium. Several hypural bones attached to last vertebra. For additional characters, see Svetovidov, 1937, Marshall & Cohen, 1973, Paulin, 1983, and several authors in Cohen, 1989. Colour: brown to black; some species pink or reddish or with silvery areas. Habitat, Distribution and Biology : Benthopelagic to pelagic species ranging from shallow coastal areas (occasionally even estuaries) to deep waters (beyond 2 500 m). -

Mcmillan NZ Fishes Vol 2
New Zealand Fishes Volume 2 A field guide to less common species caught by bottom and midwater fishing New Zealand Aquatic Environment and Biodiversity Report No. 78 ISSN 1176-9440 2011 Cover photos: Top – Naked snout rattail (Haplomacrourus nudirostris), Peter Marriott (NIWA) Centre – Red pigfish (Bodianus unimaculatus), Malcolm Francis. Bottom – Pink maomao (Caprodon longimanus), Malcolm Francis. New Zealand fishes. Volume 2: A field guide to less common species caught by bottom and midwater fishing P. J McMillan M. P. Francis L. J. Paul P. J. Marriott E. Mackay S.-J. Baird L. H. Griggs H. Sui F. Wei NIWA Private Bag 14901 Wellington 6241 New Zealand Aquatic Environment and Biodiversity Report No. 78 2011 Published by Ministry of Fisheries Wellington 2011 ISSN 1176-9440 © Ministry of Fisheries 2011 McMillan, P.J.; Francis, M.P.; Paul, L.J.; Marriott, P.J; Mackay, E.; Baird, S.-J.; Griggs, L.H.; Sui, H.; Wei, F. (2011). New Zealand fishes. Volume 2: A field guide to less common species caught by bottom and midwater fishing New Zealand Aquatic Environment and Biodiversity Report No.78. This series continues the Marine Biodiversity Biosecurity Report series which ended with MBBR No. 7 in February 2005. CONTENTS PAGE Purpose of the guide 4 Organisation of the guide 4 Methods used for the family and species guides 5 Data storage and retrieval 7 Acknowledgments 7 Section 1: External features of fishes and glossary 9 Section 2: Guide to families 15 Section 3: Guide to species 31 Section 4: References 155 Index 1 – Alphabetical list of family -

New Records of the Slender Codling Halargyreus Johnsonii Gunther, 1862 • Hoff 63
New Records of the Slender Codling Halargyreus johnsonii Gunther, 1862 • Hoff 63 New Records of the Slender Codling Halargyreus johnsonii Günther, 1862 from the Eastern Bering Sea, Alaska Gerald R. Hoff Reprinted from the Alaska Fishery Research Bulletin Vol. 9 No. 1, Summer 2002 The Alaska Fisheries Research Bulletin can be found on the World Wide Web at URL: http://www.state.ak.us/adfg/geninfo/pubs/afrb/afrbhome.htm 64 Notes NewAlaska Records Fishery of Research the Slender Bulletin Codling 9(1):6 Halargyreus5–67. 2002 johnsonii Gunther, 1862 • Hoff 65 New Records of the Slender Codling Halargyreus johnsonii Günther, 1862 from the Eastern Bering Sea, Alaska Gerald R. Hoff ABSTRACT: Two specimens of the slender codling Halargyreus johnsonii Günther, 1862 were collected from the eastern Bering Sea, the most northerly records from the eastern North Pacific. The two immature specimens were collected in June of 2000 by bottom trawl during the Alaska Fisheries Science Center’s groundfish survey of the eastern Bering Sea upper continental slope. These two individuals appear similar to all previously reported speci- mens from the Pacific and extend the range for the species to Alaska. Also reported herein are 5 previously unreported records of H. Johnsonii collected just south of the Gulf of Alaska in the eastern North Pacific. INTRODUCTION Pacific (Cohen 1973; Paulin 1983), western North Pa- cific (Kanayama et al. 1978), and eastern North Pa- The slender codling Halargyreus johnsonii Günther, cific from central California to British Columbia 1862 is the single member of the genus in the family (Logan et al. -

New Record of Gadella Jordani and Redescription of Physiculus Japonicus (Pisces: Moridae) in Korea
Anim. Syst. Evol. Divers. Vol. 32, No. 1: 28-37, January 2016 http://dx.doi.org/10.5635/ASED.2016.32.1.028 New Record of Gadella jordani and Redescription of Physiculus japonicus (Pisces: Moridae) in Korea Seo Ha Jang1, Jin-Koo Kim1,*, Jeong-Ho Park2, Young Sun Song1 1Department of Marine Biology, Pukyong National University, Busan 48513, Korea 2National Institute of Fisheries Science, Busan 46083, Korea ABSTRACT We describe the morphological characteristics of two morids, Gadella jordani and Physiculus japonicus, belonging to the order Gadiformes, based on Korean specimens collected from the Korean ocean. Two specimens of Gadella jordani was first collected from Jeju Island, Korea and the East Sea, Korea, in 2013-2014. This species is characterized by 8, 67-69 dorsal fin rays, 66-71 anal fin rays, 5+13 gill rakers, no barbel on the lower jaw, no vomerine teeth, and a ventral luminous organ closer to the anus than to the interventral line. We described it as the first record to the Korean fish fauna, and proposed the new Korean name “Min-su-yeom-dae-gu-sok” for the genus Gadella, and “Min-su-yeom-dae-gu” for the species G. jordani. Physiculus japonicus was first reported by Koh and Moon in the year 1999 based on a single specimen in Korea. However, no study has been attempted to describe the morphological characteristics in Korea since then. In 2013-2014, three specimens of P. japonicus was collected from Jeju Island, Korea and the East Sea, Korea, and we redescribe P. japonicus in detail. This species is characterized by 9-10, 63-64 dorsal fin rays, 70-73 anal fin rays, 3+7-8 gill rakers, a short barbel on the lower jaw, and a ventral luminous organ equidistant between the interventral line and the anus. -

(Actinopterygii: Gadiformes: Moridae) from Southern Japan, with a Note on the Color in Life
Species Diversity, 2010, 15, 131–138 Description of a Pelagic Juvenile Specimen of Gadella jordani (Actinopterygii: Gadiformes: Moridae) from Southern Japan, with a Note on the Color in Life Makoto Okamoto1, Kouji Matsuda2 and Tamaki Matsuda2 1 Seikai National Fisheries Research Institute, 1551-8 Taira-machi, Nagasaki, 851-2213 Japan E-mail: [email protected] 2 Scuba Diving Shop SB, 7641 Shimofukumoto-machi, Kagoshima, 891-0144 Japan (Received 1 July 2010; Accepted 14 September 2010) A pelagic juvenile (43.0 mm standard length) of the morid Gadella jordani (Böhlke and Mead, 1951) was collected from Kagoshima Prefecture, southern Japan. It has a characteristically elongated body, long dorsal and anal fin bases with 73 rays in each fin, the anus located more anteriorly than the origin of the second dorsal fin, a ventral light organ, and no chin barbel. We describe the morphology of this specimen and also present a color photo- graph of it in life. This is the first report of any early life stage in this species. Key Words: Teleostei, Gadiformes, Moridae, Gadella jordani, pelagic juve- nile, color in life, Kagoshima Prefecture, Japan. Introduction Gadella Lowe, 1843 is a genus of morid cod with members distributed from temperate to tropical regions in the deep sea (usually deeper than 150 m) of almost all oceans. They are mainly characterized by having two dorsal fins, a ventral light organ, and no chin barbel (Paulin 1989a, b; Trunov 1992; Paulin and Roberts 1997; Long and McCosker 1998; Sazonov and Shcherbachev 2000). The taxonomy of the genus was reviewed by Paulin (1989b) and Sazonov and Shcherbachev (2000), and 13 species are regarded as valid. -

Distribution and Biology of Blue Hake (Antimora Rostrata Gunther 1878) in the Northwest Atlantic with Comparison to Adjacent Areas
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE AUTHOR(S) Northwest Atlantic Fisheries Organization Serial No. N4575 NAFO SCR Doc. 01/185 SCIENTIFIC COUNCIL MEETING – SEPTEMBER 2001 Distribution and Biology of Blue Hake (Antimora rostrata Gunther 1878) in the Northwest Atlantic with Comparison to Adjacent Areas by D. W. Kulka, M. R. Simpson and T. D. Inkpen Department of Fisheries and Oceans P O Box 5667, St. John’s, Newfoundland, Canada A1C 5X1 Abstract Blue hake (Antimora rostrata) is a globally distributed species found in most slope waters around the world Based on commercial fisheries data (it was found to be a common bycatch) and research survey records, this study examines the distribution and aspects of the biology of blue hake in Canadian waters. It forms a continuous distribution in slope waters from the US/Canada border in the south (contiguous with the distribution in US slope waters) to Arctic waters between Greenland and Baffin Island. In relation to depth, blue hake were found as shallow as 200 m but rare in less than 500 m. Only 9% of the survey sets containing blue hake occurred in less than 500 m but were most common in the deepest survey sets (1600 m). Longline sets from the 1960’s at 2000-2400 m revealed that blue hake were relatively common at those depths. Studies from other parts of the world found blue hake distributed as deep as about 3000 m. Numbers per tow increased with depth, peaking at 1400 m (although depths greater than 1400 m were poorly sampled). This compares with peak abundance observed at depth of about 1700 m in US waters.