For Laboratory Grown Frost Flowers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Remote Sensing of Sea Ice
Remote Sensing of Sea Ice Peter Lemke Alfred Wegener Institute for Polar and Marine Research Bremerhaven Institute for Environmental Physics University of Bremen Contents 1. Ice and climate 2. Sea Ice Properties 3. Remote Sensing Techniques Contents 1. Ice and climate 2. Sea Ice Properties 3. Remote Sensing Techniques The Cryosphere Area Volume Volume [106 km2] [106 km3] [relativ] Ice Sheets 14.8 28.8 600 Ice Shelves 1.4 0.5 10 Sea Ice 23.0 0.05 1 Snow 45.0 0.0025 0.05 Climate System vHigh albedo vLatent heat vPlastic material Role of Ice in Climate v Impact on surface energy balance (global sink) ! Atmospheric circulation ! Oceanic circulation ! Polar amplification v Impact on gas exchange between the atmosphere and Earth’s surface v Impact on water cycle, water supply v Impact on sea level (ice mass imbalance) v Defines boundary conditions for ecosystems Role of Ice in Climate v Polar amplification in CO2 warming scenarios (surface energy balance; temperature – ice albedo feedback) IPCC, 2007 Role of Ice in Climate Polar amplification GFDL model 12 IPCC AR4 models Warming from CO2 doubling with Warming at CO2 doubling (years fixed albedo (FA) and with surface 61-80) (Winton, 2006) albedo feedback included (VA) (Hall, 2004) Contents 1. Ice and climate 2. Sea Ice Properties 3. Remote Sensing Techniques Pancake Ice Pancake Ice Old Pancake Ice First Year Ice Pressure Ridges Pressure Ridges New Ice Formation in Leads New Ice Formation in Leads New Ice Formation in Leads Melt Ponds on Arctic Sea Ice Melt ponds in the Arctic 150 m Sediment -

East Antarctic Sea Ice in Spring: Spectral Albedo of Snow, Nilas, Frost Flowers and Slush, and Light-Absorbing Impurities in Snow
Annals of Glaciology 56(69) 2015 doi: 10.3189/2015AoG69A574 53 East Antarctic sea ice in spring: spectral albedo of snow, nilas, frost flowers and slush, and light-absorbing impurities in snow Maria C. ZATKO, Stephen G. WARREN Department of Atmospheric Sciences, University of Washington, Seattle, WA, USA E-mail: [email protected] ABSTRACT. Spectral albedos of open water, nilas, nilas with frost flowers, slush, and first-year ice with both thin and thick snow cover were measured in the East Antarctic sea-ice zone during the Sea Ice Physics and Ecosystems eXperiment II (SIPEX II) from September to November 2012, near 658 S, 1208 E. Albedo was measured across the ultraviolet (UV), visible and near-infrared (nIR) wavelengths, augmenting a dataset from prior Antarctic expeditions with spectral coverage extended to longer wavelengths, and with measurement of slush and frost flowers, which had not been encountered on the prior expeditions. At visible and UV wavelengths, the albedo depends on the thickness of snow or ice; in the nIR the albedo is determined by the specific surface area. The growth of frost flowers causes the nilas albedo to increase by 0.2±0.3 in the UV and visible wavelengths. The spectral albedos are integrated over wavelength to obtain broadband albedos for wavelength bands commonly used in climate models. The albedo spectrum for deep snow on first-year sea ice shows no evidence of light- absorbing particulate impurities (LAI), such as black carbon (BC) or organics, which is consistent with the extremely small quantities of LAI found by filtering snow meltwater. -

Lecture 4: OCEANS (Outline)
LectureLecture 44 :: OCEANSOCEANS (Outline)(Outline) Basic Structures and Dynamics Ekman transport Geostrophic currents Surface Ocean Circulation Subtropicl gyre Boundary current Deep Ocean Circulation Thermohaline conveyor belt ESS200A Prof. Jin -Yi Yu BasicBasic OceanOcean StructuresStructures Warm up by sunlight! Upper Ocean (~100 m) Shallow, warm upper layer where light is abundant and where most marine life can be found. Deep Ocean Cold, dark, deep ocean where plenty supplies of nutrients and carbon exist. ESS200A No sunlight! Prof. Jin -Yi Yu BasicBasic OceanOcean CurrentCurrent SystemsSystems Upper Ocean surface circulation Deep Ocean deep ocean circulation ESS200A (from “Is The Temperature Rising?”) Prof. Jin -Yi Yu TheThe StateState ofof OceansOceans Temperature warm on the upper ocean, cold in the deeper ocean. Salinity variations determined by evaporation, precipitation, sea-ice formation and melt, and river runoff. Density small in the upper ocean, large in the deeper ocean. ESS200A Prof. Jin -Yi Yu PotentialPotential TemperatureTemperature Potential temperature is very close to temperature in the ocean. The average temperature of the world ocean is about 3.6°C. ESS200A (from Global Physical Climatology ) Prof. Jin -Yi Yu SalinitySalinity E < P Sea-ice formation and melting E > P Salinity is the mass of dissolved salts in a kilogram of seawater. Unit: ‰ (part per thousand; per mil). The average salinity of the world ocean is 34.7‰. Four major factors that affect salinity: evaporation, precipitation, inflow of river water, and sea-ice formation and melting. (from Global Physical Climatology ) ESS200A Prof. Jin -Yi Yu Low density due to absorption of solar energy near the surface. DensityDensity Seawater is almost incompressible, so the density of seawater is always very close to 1000 kg/m 3. -

Interannual Variability in Transpolar Drift Ice Thickness and Potential Impact of Atlantification
https://doi.org/10.5194/tc-2020-305 Preprint. Discussion started: 22 October 2020 c Author(s) 2020. CC BY 4.0 License. Interannual variability in Transpolar Drift ice thickness and potential impact of Atlantification H. Jakob Belter1, Thomas Krumpen1, Luisa von Albedyll1, Tatiana A. Alekseeva2, Sergei V. Frolov2, Stefan Hendricks1, Andreas Herber1, Igor Polyakov3,4,5, Ian Raphael6, Robert Ricker1, Sergei S. Serovetnikov2, Melinda Webster7, and Christian Haas1 1Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany 2Arctic and Antarctic Research Institute, St. Petersburg, Russian Federation 3International Arctic Research Center, University of Alaska Fairbanks, Fairbanks, US 4College of Natural Science and Mathematics, University of Alaska Fairbanks, Fairbanks, US 5Finnish Meteorological Institute, Helsinki, Finland 6Thayer School of Engineering at Dartmouth College, Hanover, US 7Geophysical Institute, University of Alaska Fairbanks, Fairbanks, US Correspondence: H. Jakob Belter ([email protected]) Abstract. Changes in Arctic sea ice thickness are the result of complex interactions of the dynamic and variable ice cover with atmosphere and ocean. Most of the sea ice exits the Arctic Ocean through Fram Strait, which is why long-term measurements of ice thickness at the end of the Transpolar Drift provide insight into the integrated signals of thermodynamic and dynamic influences along the pathways of Arctic sea ice. We present an updated time series of extensive ice thickness surveys carried 5 out at the end of the Transpolar Drift between 2001 and 2020. Overall, we see a more than 20% thinning of modal ice thickness since 2001. A comparison with first preliminary results from the international Multidisciplinary drifting Observatory for the Study of Arctic Climate (MOSAiC) shows that the modal summer thickness of the MOSAiC floe and its wider vicinity are consistent with measurements from previous years. -
Initially, the Committee's Work Focused on the Hydraulic Aspects of River Ice
Ice composites as construction materials in projects of ice structures Citation for published version (APA): Vasiliev, N. K., & Pronk, A. D. C. (2015). Ice composites as construction materials in projects of ice structures. 1- 11. Paper presented at 23rd International Conference on Port and Ocean Engineering under Arctic Conditions (POAC ’15), June 14-18, 2015, Trondheim, Norway, Trondheim, Norway. Document status and date: Published: 14/06/2015 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Understanding Earth's Cold Regions
National Aeronautics and Space Administration Proposed NASA–ISRO SAR Mission Understanding Earth’s Cold Regions NISAR will measure changes in glacier and ice sheet motion, sea ice, and permafrost to determine how global climate and ice masses interrelate and how melting of land ice raises sea level. Impacts of Earth’s Remote Ice Perhaps you imagine the polar ice sheets as icy white blankets at the Snow Cover ends of the Earth, static and majestic, but far removed from your daily life. In reality, these areas are among the Sea most dynamic and rapidly changing Ice Ice Shelf places on Earth, where wind and Ice Sheet currents move ice over the seas, Glaciers and the forces of gravity disgorge Sea huge icebergs to the ocean. These Level Rise distant changes have very real local consequences of climate feedbacks Lake and Frozen and rising sea level. River Ice Ground Assessing Society’s Exposure to Diminishing Ice ice cover is decreasing drastically and may vanish entirely within the next decades. The loss of sea ice cover will have a For over a hundred years, scientists have considered diminishing profound effect on life, climate, and commercial activities in the glaciers and sea ice to be an early indicator of climate change. Arctic, while the loss of land ice will impact an important source At the same time, ice sheets and glaciers are already melting of water for millions of people. Collectively, these effects mean fast enough to be the largest contributors to sea level rise, with that despite its remote location, changes in ice have global a potential to raise sea level by several tens of centimeters or economic and health implications as climate changes. -

Crusty Alga Uncovers Sea-Ice Loss That Inhibits COX-2
Selections from the RESEARCH HIGHLIGHTS scientific literature PHARMACOLOGY Painkiller kills the bad effects of pot Marijuana’s undesirable effects NICK CALOYIANIS on the brain can be overcome by using painkillers similar to ibuprofen, at least in mice. Chu Chen and his colleagues at Louisiana State University Health Sciences Center in New Orleans treated mice with THC, marijuana’s main active ingredient. They found that THC impaired the animals’ memory and the efficiency of their neuronal signalling, probably by stimulating the enzyme COX-2. The authors reversed these negative effects — and were able to maintain marijuana’s benefits, such as reducing CLIMATE SCIENCES neurodegeneration — when they also treated the mice with a drug, similar to ibuprofen, Crusty alga uncovers sea-ice loss that inhibits COX-2. The authors suggest that the Like tree rings, layers of growth in a long-lived marine alga Clathromorphum compactum benefits of medical marijuana Arctic alga may preserve a temperature record (pictured). It can live for hundreds of years and could be enhanced with the use of past climate. Specimens from the Canadian builds a fresh layer of crust each year. of such inhibitors. Arctic indicate that sea-ice cover has shrunk The thickness of each layer, and the ratio of Cell 155, 1154–1165 (2013) drastically in the past 150 years — to the lowest magnesium to calcium within it, are linked to levels in the 646 years of the algal record. water temperature and the amount of sunlight PALAEOECOLOGY Satellite records of the Arctic’s shrinking the organism receives. The discovery suggests sea-ice cover date back only to the late 1970s. -

Article Sources and Dynamics, Other Seasons (Ducklow Et Al., 2015; Kim Et Al., 2015, 2019)
Biogeosciences, 16, 2683–2691, 2019 https://doi.org/10.5194/bg-16-2683-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Collection of large benthic invertebrates in sediment traps in the Amundsen Sea, Antarctica Minkyoung Kim1, Eun Jin Yang2, Hyung Jeek Kim3, Dongseon Kim3, Tae-Wan Kim2, Hyoung Sul La2, SangHoon Lee2, and Jeomshik Hwang1 1School of Earth and Environmental Sciences/Research Institute of Oceanography, Seoul National University, Seoul, 08826, South Korea 2Korea Polar Research Institute, Incheon, 21990, South Korea 3Korea Institute of Ocean Science and Technology, Busan, 49111, South Korea Correspondence: Jeomshik Hwang ([email protected]) Received: 18 February 2019 – Discussion started: 6 March 2019 Revised: 4 June 2019 – Accepted: 18 June 2019 – Published: 11 July 2019 Abstract. To study sinking particle sources and dynamics, other seasons (Ducklow et al., 2015; Kim et al., 2015, 2019). sediment traps were deployed at three sites in the Amund- Biogeochemical processes related to biological pump in the sen Sea for 1 year from February–March 2012 and at one Amundsen Sea have been investigated by recent field cam- site from February 2016 to February 2018. Unexpectedly, paigns (Arrigo and Alderkamp, 2012; Yager et al., 2012; large benthic invertebrates were found in three sediment traps Meredith et al., 2016; Lee et al., 2017). deployed 130–567 m above the sea floor. The organisms in- Sediment traps were deployed in the Amundsen Sea to cluded long and slender worms, a sea urchin, and juvenile study sinking material flux and composition. Sampling oc- scallops of varying sizes. -

Can Sea Water Freeze?
Can Sea Water Freeze? Unit: Salinity Patterns & the Water Cycle l Time Required: Four 45 min. class period l Grade Level: Middle l Content Standard: NSES Physical Science, properties and changes of properties in matter Ocean Literacy Principle 1e: Most of Earth's water (97%) is in the ocean. Seawater has unique properties: it is saline, its freezing point is slightly lower than fresh water, its density is slightly higher, its electrical conductivity is much higher, and it is slightly basic. Big Idea: Salt causes water to freeze at a lower temperature. Sea ice forms only under very cold conditions (e.g., near Earth’s poles) because of its salinity (i.e., presence of dissolved salt). Key Concepts: • Dissolving any substance in pure water raises or lowers the freezing and boiling point. • When water freezes - goes from the liquid state to the solid state - its molecules go from a disorganized state to an organized state. • When water freezes to a solid, molecular motion slows down enough that the molecules become permanently fixed in an orderly arrangement called a crystal. • The individual particles that make up salt (known as ions) arrange themselves around the water molecules. In doing so, they shield the water molecules from interactions among themselves, making it less likely that they will find each other and form ice. • The water molecules have to be slowed down even more in the presence of salt in order to form a solid. So you have to go to a lower temperature in order to freeze water that contains salt. • Salt is excluded in the formation of ice; therefore ice made from salt water is essentially salt-free. -

Unit 3. Antarctic Oceanography Lesson 1
ANTARCTIC Unit 3. Antarctic Oceanography Lesson 1. – Overview and Research Stations Lesson Objectives: • Introduces the continent of Antarctica and the oceans that surround it • The student will learn about the geography, history and climate. • The second section of this chapter discusses research stations and the scientists who live on the frozen continent. Antarctica is a continent located form 25 million years ago. The at the southern-most point of ice in Antarctica locks up more the globe. Millions of years ago, than two-thirds of the planet's this landmass was attached to fresh water. If the Antarctic ice a giant landmass that consisted were to melt, the sea would rise of modern-day South America, almost 200 feet. It is the only India and Africa. Powerful underground forces ripped a large piece of land from this giant landmass, which then drifted to its current position at the bottom of the globe. It is surrounded on all sides by the Indian, Pacific and Atlantic Oceans. continent that man had left untouched for Antarctica's cold, thick hard millions of years. covering, called an Antarctica is ice considered the sheet, coldest and driest continent on began to earth. Temperatures decrease regions inland. Temperatures as one moves from the coastal during the long, dark winters ©PROJECT OCEANOGRAPHY ANTARCTIC OCEANOGRAPHY 87 ANTARCTIC range from –4° F to –22° F on Blizzards are produced not by the coast, -40° F to –90° F falling snow, but when high inland. During the summers, winds (100- 200mph) blow coastal temperatures average ground snow around, creating 32° F (occasionally climbing to blinding conditions and 50° F), while the inland summer snowdrifts that can cover local temperatures range from –4° F research stations in an hour. -

Anchor Ice and Bottom-Freezing in High-Latitude Marine Sedimentary Environments: Observations from the Alaskan Beaufort Sea
ANCHOR ICE AND BOTTOM-FREEZING IN HIGH-LATITUDE MARINE SEDIMENTARY ENVIRONMENTS: OBSERVATIONS FROM THE ALASKAN BEAUFORT SEA by Erk Reimnitz, E. W. Kempema, and P. W. Barnes U.S. Geological Survey Menlo Park, California 94025 Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 205 1986 257 This report has also been published as U.S. Geological Survey Open-File Report 86-298. ACKNOWLEDGMENTS This study was funded in part by the Minerals Management Service, Department of the Interior, through interagency agreement with the National Oceanic and Atmospheric Administration, Department of Commerce, as part of the Alaska Outer Continental Shelf Environmental Assessment Program. We thank D. A. Cacchione for his thoughtful review of the manuscript. TABLE OF CONTENTS Page ACKNOWLEDGMENTS . ...259 INTRODUCTION . 263 REGIONAL SETTING . ...264 INDIRECT EVIDENCE FOR ANCHOR ICE IN THE BEAUFORT SEA . ...266 DIVER OBSERVATIONS OF ANCHOR ICE AND ICE-BONDED SEDIMENTS . ...271 DISCUSSION AND CONCLUSION . ...274 REFERENCES CITED . ...278 INTRODUCTION As early as 1705 sailors observed that rivers sometimes begin to freeze from the bottom (Barnes, 1928; Piotrovich, 1956). Anchor ice has been observed also in lakes and the sea (Zubov, 1945; Dayton, et al., 1969; Foulds and Wigle, 1977; Martin, 1981; Tsang, 1982). The growth of anchor ice implies interactions between ice and the sub- strate, and a marked change in the sedimentary environment. However, while the literature contains numerous observations that imply sediment transport, no studies have been conducted on the effects of anchor ice growth on sediment dynamics and bedforms. Underwater ice is the general term for ice formed in the supercooled water column. -
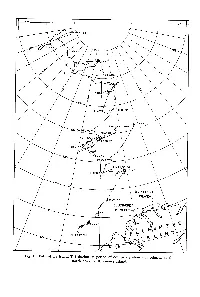
ARCTIC ICE ISLAND and ICE SHELF STUDIES Part I
Fig. 1. Path of ice island T-3 during its period of occupancy shown in relation to the north coast of Ellesmere Island. ARCTIC ICE ISLAND AND ICE SHELF STUDIES Part I A. P. Crary* Introduction N March 1952, in what was termed Project ICICLE, under the leader- I ship of Lt. Col. Joseph 0. Fletcher, the Alaskan Air Command of the United States Air Force landed a small party of men about 150 miles from the North Pole on T-3, one of three floating ice islands whose movements in the Arctic Ocean had been followed since the first sighting in 1946. Soon after the initial landings meteorological upper air observations were started by the Air Weather Service, and geophysical studies were begun by theAir Force Cambridge Research Center. Theupper air weather station continued in operation for a total of 22 months until in 1954 at 84"40'N., 81"W. operations were abandoned. During this period of occu- pation various scientific studies were carried out periodically by personnel from the Air Force Cambridge Research Center, from the University of Southern California under contract to the Arctic Aeromedical Laboratory, from Woods Hole Oceanographic Institute under contract to the Office of Naval Research, and from SIPRE (Snow, Ice, and Permafrost Research Establishment). A small group of scientists reoccupied the island from April to September 1955 when it was in thegeneral area of 83"N., 90"W. In an attempt to correlate the character of the ice island with that of the Ellesmere Ice Shelf, the presumed source of T-3, two expeditions led by Geoffrey Hattersley-Smith of the Defence Research Board of Canada explored the northern shores of Ellesmere Island in the summers of 1953 and 1954 from the weather station Alert to Lands Lokk, with the main efforts being concentrated near Ward Hunt Island in 1954.