Direct Determination of Cyanate in a Urea Solution and a Urea-Containing Protein Buffer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Second Tranche HTS Subheading Product Description 2710.19.30
Second Tranche HTS Subheading Product Description 2710.19.30 Lubricating oils, w/or w/o additives, fr. petro oils and bitumin minerals (o/than crude) or preps. 70%+ by wt. fr. petro oils 2710.19.35 Lubricating greases from petro oil/bitum min/70%+ by wt. fr. petro. oils but n/o 10% by wt. of fatty acid salts animal/vegetable origin 2710.19.40 Lubricating greases from petro oil/bitum min/70%+ by wt. fr. petro. oils > 10% by wt. of fatty acid salts animal/vegetable origin 3403.19.10 Lubricating preparations containing 50% but less than 70% by weight of petroleum oils or of oils obtained from bituminous minerals 3403.19.50 Lubricating preparations containing less than 50% by weight of petroleum oils or of oils from bituminous minerals 3403.99.00 Lubricating preparations (incl. lubricant-based preparations), nesoi 3811.21.00 Additives for lubricating oils containing petroleum oils or oils obtained from bituminous minerals 3811.29.00 Additives for lubricating oils, nesoi 3901.10.10 Polyethylene having a specific gravity of less than 0.94 and having a relative viscosity of 1.44 or more, in primary forms 3901.10.50 Polyethylene having a specific gravity of less than 0.94, in primary forms, nesoi 3901.20.10 Polyethylene having a specific gravity of 0.94 or more and having a relative viscosity of 1.44 or more, in primary forms 3901.20.50 Polyethylene having a specific gravity of 0.94 or more, in primary forms, nesoi 3901.30.20 Ethylene copolymer: Vinyl acetate-vinyl chloride-ethylene terpoly w/ < 50% deriv of vinyl acetate, exc polymer aromatic/mod -

TR-470: Pyridine (CASRN 110-86-1) in F344/N Rats, Wistar Rats, And
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF PYRIDINE (CAS NO. 110-86-1) IN F344/N RATS, WISTAR RATS, AND B6C3F1 MICE (DRINKING WATER STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 March 2000 NTP TR 470 NIH Publication No. 00-3960 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Technical Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. -

Heats of Formation of Certain Nickel-Pyridine Complex Salts
HEATS OF FORFATION OF CERTAIN NICKEL-PYRIDINE COLPLEX SALTS DAVID CLAIR BUSH A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE June l9O [4CKNOWLEDGMENT The writer wishes to acknowledge his indebtedness and gratitude to Dr. [4. V. Logan for his help and encour- agement during this investigation. The writer also wishes to express his appreciation to Dr. E. C. Gilbert for helpful suggestions on the con- struction of the calortheter, and to Lee F. Tiller for his excellent drafting and photostating of the figures and graphs. APPROVED: In Charge of ?ajor Head of Department of Chemistry Chairrian of School Graduate Comrittee Dean of Graduate School Date thesis is presented /11 ' Typed by Norma Bush TABLE OF CONTENTS HISTORICAL BACKGROUND i INTRODUCTION 2 EXPERIMENTAL 5 Preparation of the Compounds 5 Analyses of the Compounds 7 The Calorimeter Determination of the Heat Capacity 19 Determination of the Heat of Formation 22 DISCUSSION 39 41 LITERATURE CITED 42 TABLES I Analyses of the Compounds 8 II Heat Capacity of the Calorimeter 23 III Heat of Reaction of Pyridine 26 IV Sample Run and Calculation 27 V Heat of Reaction of Nickel Cyanate 30 VI Heat of Reaction of Nickel Thiocyanate 31 VII Heat of Reaction of Hexapyridinated Nickel Cyanate 32 VIII Heat of Reaction of Tetrapyridinated Nickel Thiocyanate 33 IX Heat of Formation of the Pyridine Complexes 34 FI GURES i The Calorimeter 10 2 Sample Ijector (solids) 14 2A Sample Ejector (liquids) 15 3 Heater Circuit Wiring Diagram 17 4 heat Capacity of the Calorimeter 24 5 Heat of Reaction of Pyridine 28 6 Heat of Reaction of Hexapyridinated Nickel Cyanate 35 7 Heat of Reaction of Tetrapyridinated Nickel Thiocyanate 36 8 Heat of Reaction of Nickel Cyanate 37 9 Heat of Reaction of Nickel Thiocyanate 38 HEATS OF FOW ATION OF CERTAIN NICKEL-PYRIDINE COMPLEX SALTS HISTORICAL BACKGROUND Compounds of pyridine with inorganic salts have been prepared since 1970. -

WO 2014/185925 Al 20 November 2014 (20.11.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/185925 Al 20 November 2014 (20.11.2014) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BOW 53/28 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US20 13/04 1503 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 17 May 2013 (17.05.2013) NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (25) Filing Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (26) Publication Language: English ZM, ZW. (71) Applicant: EMPIRE TECHNOLOGY DEVELOP¬ (84) Designated States (unless otherwise indicated, for every MENT LLC [US/US]; 271 1 Centerville Road, Suite 400, kind of regional protection available): ARIPO (BW, GH, Wilmington, Delaware 19808 (US). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: PEPPOU, George Charles; 4 Armen Way, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Hornsby Heights, New South Wales 2077 (AU). -

Intramolecular Alkoxycyanation and Alkoxyacylation Reactions: New Types of Alkene Difunctionalizations for the Construction of Oxygen Heterocycles John P
Angewandte. Highlights DOI: 10.1002/anie.201204470 Alkene Difunctionalization Intramolecular Alkoxycyanation and Alkoxyacylation Reactions: New Types of Alkene Difunctionalizations for the Construction of Oxygen Heterocycles John P. Wolfe* alkenes · catalysis · heterocycles · ketones · nitriles Saturated oxygen heterocycles, such as tetrahydrofurans and not require an exogenous carbon electrophile.[6,7] Instead, the dihydrobenzofurans, are important motifs in a myriad of carbon electrophile is covalently attached to the cyclizing biologically active compounds, including natural products and oxygen atom in the substrate, and the carboalkoxylations are pharmaceutical targets. Therefore, there has been consider- accomplished through activation of this CÀO bond by the able interest in the development of new synthetic methods for catalyst. Importantly, these two approaches lead to the the construction of these important structures.[1] Many tradi- formation of dihydrobenzofuran derivatives that bear func- tional approaches to the generation of these compounds tional groups (ketones or nitriles), which are convenient involve ring formation through intramolecular SN2 reactions handles for further elaboration of the molecule, and cannot be and related strategies. However, these approaches typically directly installed by using previously developed alkene lead to the formation of only one bond during ring closure and carboalkoxylation methods. often require somewhat complicated substrates.[1] The method of the Douglas group involves the use of Late transition metal catalyzed alkene carboalkoxylations a cationic RhI complex to catalyze the intramolecular are a subclass of alkene difunctionalization reactions[2] that alkoxyacylation of acylated 2-allylphenol derivatives have considerable utility for the construction of tetrahydro- (Scheme 2).[6] These reactions afford 2-acylmethyl dihydro- furans, dihydrobenzofurans, and related oxygen heterocycles. -
Investigating Cell Type Specific Mechanisms Contributing to Acute Oral Toxicity
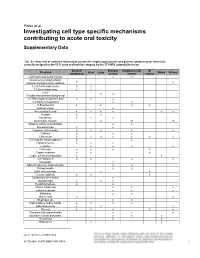
Prieto et al.: Investigating cell type specific mechanisms contributing to acute oral toxicity Supplementary Data1 Tab. S1: Overview of collected information on specific target organ/system and general cytotoxicity for chemicals correctly assigned to the CLP acute oral toxicity category by the 3T3 NRU cytotoxicity assay General Nervous Cardiovascular GI Chemical Liver Lung Blood Kidney cytotoxicity system system system (±)-Propranolol hydrochloride x ax (4-ammonio-m-tolyl)ethyl(2- x x hydroxyethyl)ammonium sulphate 1,2,4-Trichlorobenzene x x 1,2-Dichlorobenzene x x 2,4,6- x x Tris(dimethylaminomethyl)phenol 2,4-Dichlorophenoxyacetic acid x x x 5,5-Diphenylhydantoin x x 5-Fluorouracil x x x x Acetophenone x Acetylsalicylic acid x x x x x x Acrolein x x Acrylamide x x x Ammonium chloride x ax ax Atropine sulfate monohydrate x x Benzaldehyde x x Cadmium (III) chloride x x x x x Caffeine x x x Chloroform x x ax x x x x Chloroquine bis(phosphate) x x Chlorpromazine x x x Codeine x x x x Colchicine x x x x Copper sulphate x x x Cupric sulfate pentahydrate x x Cyclosporin A x x x x Diazepam x Diphenhydramine hydrochloride x x Disopyramide x x Ethyl chloroacetate x x Ferrous sulphate x x x Glufosinate-ammonium x Glutethimide x ax x Hexachlorophene x x Lithium Carbonate x x x Lithium sulphate x x x Malathion x x Maleic acid x x Meprobamate x x Orphenadrine hydrochloride x x x x p-Benzoquinone x x x Phenol x x x x x Procainamide hydrochloride x x x Quinidine sulfate dehydrate x x x Resorcinol x x Rifampicin x x doi:10.14573/altex.1805181s2 ALTEX ##(#), SUPPLEMENTARY DATA 1 General Nervous Cardiovascular GI Chemical Liver Lung Blood Kidney cytotoxicity system system system Sodium Cyanate x x sodium oxalate x x x Sodium valproate x bx x x Thioridazine hydrochloride x x Valproic acid x x x x x GI: Gastrointestinal; CLP: Classification, labelling and packaging; NRU: Neutral Red Uptake; a Indirect effect: b chronic effect Tab. -

Sodium Cyanate Manufaturer… TIRUPATI CYANATE
Sodium Cyanate Manufaturer… TIRUPATI CYANATE www.tirupaticyanate.com ABOUT US___________________________________________________ Tirupati cyanate is leading manufacturer and provider of sodium cyanate. Our excellent customer service and high quality of Sodium cyanate in bulk makes us unique in the market. Tirupati cyanate is exporters of sodium cyanate in volumes to supply to the demands of its customers in local market and around the world. We undertake custom synthesis and manufacture sodium cyanate as per demand and under secrecy agreement. Please enquire and contact us for further information on sodium cynate mentioning your specific requirements. Sodium Cyanate Specification CAS No 917-61-3 Cyanic Acid, Sodium Salt, Natriumcyanat 9, Synonym Cianato de sodio, Cyanate de sodium Molecular Weight 65.01 Empirical Formula NaOCn Sodium Cyanate Physical property Purity Minimum 90% Melting Point 550°C Density 1.893 g/cm3 Solubility 10 gm at 25ºC Physical Appearance Free Flowing White Powder Soda Ash Content Less Than 5% Cyanate (-OCN) Content Between 59.5 % to 61.3 % Loss on Drying (At 110ºC)Max Maximum 0.05 Packaging Information HDPE Bags inside liner of 25kgs, 50 Kgs. Capacity Company Tirupati cyanate, chhatral GIDC industrial estate, Dist. Gandhinagar, Gujarat, India Sodium cyanate Application Sodium Cyanate is mainly used as herbicides & in heat treatment of metal. As herbicides it is used mostly to wipeout weeds in lawns and onion crops. It also used as a fertilizer because sodium cyanate contains high volume of nitrogen. Copyright © TIRUPATI CYANATE 2005-2013 Sodium Cyanate Manufaturer… TIRUPATI CYANATE www.tirupaticyanate.com Sodium cyanate is also used in the synthesis of pesticides and dyes intermediates. -

United States Patent Office Patented Dec
3,631,000 United States Patent Office Patented Dec. 28, 1971 2 SUMMARY OF THE INVENTION 3,631,000 SOCYANURATE-CONTAINING POLYSOCYA We have found that superior isocyanurate-containing NATES AND METHOD OF PREPARATION polyisocyanates are inexpensively prepared by the two Perry A. Argabright and Brian L. Philips, Littleton, Colo., step process of: (1) chloroalkylating a mono-substituted and Vernon J. Sinkey, South St. Paul, Minn., assignors benzene compound, and (2) reacting the polychloro to Marathon Oil Company, Findlay, Ohio alkylated benzene-substituted compounds with a metal No Drawing. Filed June 4, 1969, Ser. No. 830,541 cyanate in the conjoint presence of a bromide or iodide Int. C. C08g 22/18, 22/44 of an alkali metal or an alkaline earth metal, and in the U.S. C. 260-77.5 NC 1. Claims presence of an aprotic solvent, herein defined. The mole IO ratio of metal cyanate to the chloride in the polychloro alkylated benzene-substituted compound is from about ABSTRACT OF THE DESCLOSURE 0.8 to about 1.5 to produce the polyisocyanates. These Improved organic polyisocyanates are prepared by re polyisocyanate compositions are starting materials for acting chlorinated benzene-substituted compounds, espe Various polymeric systems, e.g. a rigid polyurethane foam cially chloromethylated aromatics, with metal cyanates in 5 produced by conventional polymerization or copolymeri the presence of a metal iodide or bromide and in the zation with an appropriate monomer (e.g. a polyester or presence of a dipolar aprotic solvent where the mole ratio polyether based polyol). Particularly preferred products of cyanate in the metal cyanate to chlorine in the chlo are polyurethane and polyurea foams, coatings, elasto rine-containing benzene-substituted compound is from mers and adhesives. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

(12) United States Patent (10) Patent No.: US 8,877,934 B2 Berger Et Al
US008877934B2 (12) United States Patent (10) Patent No.: US 8,877,934 B2 Berger et al. (45) Date of Patent: Nov. 4, 2014 (54) N-HETEROARYL COMPOUNDS (52) U.S. Cl. CPC ............ C07D 213/89 (2013.01); C07D401/04 (75) Inventors: Michael Berger, Schwabenheim (DE); (2013.01); C07D 213/74 (2013.01); C07D Christopher Kern, Schwabenheim 239/47 (2013.01); C07D 239/42 (2013.01); (DE); Marko Eck, Schwabenheim (DE): C07D 413/04 (2013.01); C07D491/048 Jörg Schröder, Schwabenheim (DE) (2013.01); C07D 471/04 (2013.01); C07D 215/46 (2013.01) (73) Assignee: Intervet Inc., Summit, NJ (US) USPC ........................................ 546/309; 514/235.8 (58) Field of Classification Search (*) Notice: Subject to any disclaimer, the term of this None patent is extended or adjusted under 35 See application file for complete search history. U.S.C. 154(b) by 0 days. (56) References Cited (21) Appl. No.: 13/876,724 FOREIGN PATENT DOCUMENTS (22) PCT Filed: Sep. 28, 2011 CN 1.01284.812 A 10, 2008 EP 1900 772 A2 3, 2008 (86). PCT No.: WO 2004/03.5591 A1 4/2004 WO 2008/028689 A1 3, 2008 S371 (c)(1), WO 2008/028691 A1 3, 2008 (2), (4) Date: Mar. 28, 2013 OTHER PUBLICATIONS (87) PCT Pub. No.: WO2012/041873 Silva, A., Mini-Reviews in Medicinal Chemistry, 2005, vol. 5, pp. 893-1014.* PCT Pub. Date: Apr. 5, 2012 Jabbar et al., “Anthelmintic resistance: the sate of play revisited'. Life Sciences, 2006, pp. 2413-2431, vol. 79. (65) Prior Publication Data McKellar et al., “Veterinary anthelmintics: old and new”, Trends in US 2013/O196993 A1 Aug. -

Rpt POL-TOXIC AIR POLLUTANTS 98 BY
SWCAA TOXIC AIR POLLUTANTS '98 by CAS ASIL TAP SQER CAS No HAP POLLUTANT NAME HAP CAT 24hr ug/m3 Ann ug/m3 Class lbs/yr lbs/hr none17 BN 1750 0.20 ALUMINUM compounds none0.00023 AY None None ARSENIC compounds (E649418) ARSENIC COMPOUNDS none0.12 AY 20 None BENZENE, TOLUENE, ETHYLBENZENE, XYLENES BENZENE none0.12 AY 20 None BTEX BENZENE none0.000083 AY None None CHROMIUM (VI) compounds CHROMIUM COMPOUN none0.000083 AY None None CHROMIUM compounds (E649962) CHROMIUM COMPOUN none0.0016 AY 0.5 None COKE OVEN COMPOUNDS (E649830) - CAA 112B COKE OVEN EMISSIONS none3.3 BN 175 0.02 COPPER compounds none0.67 BN 175 0.02 COTTON DUST (raw) none17 BY 1,750 0.20 CYANIDE compounds CYANIDE COMPOUNDS none33 BN 5,250 0.60 FIBROUS GLASS DUST none33 BY 5,250 0.60 FINE MINERAL FIBERS FINE MINERAL FIBERS none8.3 BN 175 0.20 FLUORIDES, as F, containing fluoride, NOS none0.00000003 AY None None FURANS, NITRO- DIOXINS/FURANS none5900 BY 43,748 5.0 HEXANE, other isomers none3.3 BN 175 0.02 IRON SALTS, soluble as Fe none00 AN None None ISOPROPYL OILS none0.5 AY None None LEAD compounds (E650002) LEAD COMPOUNDS none0.4 BY 175 0.02 MANGANESE compounds (E650010) MANGANESE COMPOU none0.33 BY 175 0.02 MERCURY compounds (E650028) MERCURY COMPOUND none33 BY 5,250 0.60 MINERAL FIBERS ((fine), incl glass, glass wool, rock wool, slag w FINE MINERAL FIBERS none0.0021 AY 0.5 None NICKEL 59 (NY059280) NICKEL COMPOUNDS none0.0021 AY 0.5 None NICKEL compounds (E650036) NICKEL COMPOUNDS none0.00000003 AY None None NITROFURANS (nitrofurans furazolidone) DIOXINS/FURANS none0.0013