Scleroderma – Lupus Overlap Syndrome: Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Foot Pain in Scleroderma
Foot Pain in Scleroderma Dr Begonya Alcacer-Pitarch LMBRU Postdoctoral Research Fellow 20th Anniversary Scleroderma Family Day 16th May 2015 Leeds Institute of Rheumatic and Musculoskeletal Medicine Presentation Content n Introduction n Different types of foot pain n Factors contributing to foot pain n Impact of foot pain on Quality of Life (QoL) Leeds Institute of Rheumatic and Musculoskeletal Medicine Scleroderma n Clinical features of scleroderma – Microvascular (small vessel) and macrovascular (large vessel) damage – Fibrosis of the skin and internal organs – Dysfunction of the immune system n Unknown aetiology n Female to male ratio 4.6 : 1 n The prevalence of SSc in the UK is 8.21 per 100 000 Leeds Institute of Rheumatic and Musculoskeletal Medicine Foot Involvement in SSc n Clinically 90% of SSc patients have foot involvement n It typically has a later involvement than hands n Foot involvement is less frequent than hand involvement, but is potentially disabling Leeds Institute of Rheumatic and Musculoskeletal Medicine Different Types of Foot Pain Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Microvascular disease (small vessel) n Intermittent pain – Raynaud’s (spasm) • Cold • Throb • Numb • Tingle • Pain n Constant pain – Vessel center narrows • Distal pain (toes) • Gradually increasing pain • Intolerable pain when necrosis is present Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Macrovascular disease (large vessels) n Intermittent and constant pain – Peripheral Arterial Disease • Intermittent claudication – Muscle pain (ache, cramp) during walking • Aching or burning pain • Night and rest pain • Cramps Leeds Institute of Rheumatic and Musculoskeletal Medicine Ulcer Pain n Ulcer development – Constant pain n Infected ulcer – Unexpected/ excess pain or tenderness Leeds Institute of Rheumatic and Musculoskeletal Medicine Neuropathic Pain n Nerve damage is not always obvious. -

A Patient with Plaque Type Morphea Mimicking Systemic Lupus Erythematosus
CASE REPORT A Patient With Plaque Type Morphea Mimicking Systemic Lupus Erythematosus Wardhana1, EA Datau2 1 Department of Internal Medicine, Siloam International Hospitals. Karawaci, Indonesia. 2 Department of Internal Medicine, Prof. Dr. RD Kandou General Hospital & Sitti Maryam Islamic Hospital, Manado, North Sulawesi, Indonesia. Correspondence mail: Siloam Hospitals Group’s CEO Office, Siloam Hospital Lippo Village. 5th floor. Jl. Siloam No.6, Karawaci, Indonesia. email: [email protected] ABSTRAK Morfea merupakan penyakit jaringan penyambung yang jarang dengan gambaran utama berupa penebalan dermis tanpa disertai keterlibatan organ dalam. Penyakit ini juga dikenal sebagai bagian dari skleroderma terlokalisir. Berdasarkan gambaran klinis dan kedalaman jaringan yang terlibat, morfea dikelompokkan ke dalam beberapa bentuk dan sekitar dua pertiga orang dewasa dengan morfea mempunyai tipe plak. Produksi kolagen yang berlebihan oleh fibroblast merupakan penyebab kelainan pada morfea dan mekanisme terjadinya aktivitas fibroblast yang berlebihan ini masih belum diketahui, meskipun beberapa mekanisme pernah diajukan. Morfe tipe plak biasanya bersifat ringan dan dapat sembuh dengan sendirinya. Morfea tipe plak yang penampilan klinisnya menyerupai lupus eritematosus sistemik, misalnya meliputi alopesia dan ulkus mukosa di mulut, jarang dijumpai. Sebuah kasus morfea tipe plak pada wanita berusia 20 tahun dibahas. Pasien ini diobati dengan imunosupresan dan antioksidan local maupun sistemik. Kondisi paisen membaik tanpa disertai efek samping yang berarti. Kata kunci: morfea, tipe plak. ABSTRACT Morphea is an uncommon connective tissue disease with the most prominent feature being thickening or fibrosis of the dermal without internal organ involvement. It is also known as a part of localized scleroderma. Based on clinical presentation and depth of tissue involvement, morphea is classified into several forms, and about two thirds of adults with morphea have plaque type. -

Case Report an Exophytic and Symptomatic Lesion of the Labial Mucosa Diagnosed As Labial Seborrheic Keratosis
Int J Clin Exp Pathol 2019;12(7):2749-2752 www.ijcep.com /ISSN:1936-2625/IJCEP0093949 Case Report An exophytic and symptomatic lesion of the labial mucosa diagnosed as labial seborrheic keratosis Hui Feng1,2, Binjie Liu1, Zhigang Yao1, Xin Zeng2, Qianming Chen2 1XiangYa Stomatological Hospital, Central South University, Changsha 410000, Hunan, P. R. China; 2State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan, P. R. China Received March 16, 2019; Accepted April 23, 2019; Epub July 1, 2019; Published July 15, 2019 Abstract: Seborrheic keratosis is a common benign epidermal tumor that occurs mainly in the skin of the face and neck, trunk. The tumors are not, however, seen on the oral mucous membrane. Herein, we describe a case of labial seborrheic keratosis confirmed by histopathology. A healthy 63-year-old man was referred to our hospital for evalu- ation and treatment of a 2-month history of a labial mass with mild pain. Clinically, the initial impressions were ma- lignant transformation of chronic discoid lupus erythematosus, syphilitic chancre, or keratoacanthoma. Surprisingly, our laboratory results and histopathologic evaluations established a novel diagnosis of a hyperkeratotic type of labial seborrheic keratosis (SK). This reminds us that atypical or varying features of seborrheic keratosis make it difficult to provide an accurate diagnosis. Clinical manifestations of some benign lesions may be misdiagnosed as malignancy. Consequently, dentists should consider this as a differential diagnosis in labial or other oral lesions. Keywords: Seborrheic keratosis, exophytic lesion, symptomatic lesion, labial mucosa, oral mucous membrane Introduction findings were revealed in his medical history, and he had no known allergies to foods or me- Seborrheic keratosis is a common benign epi- dications. -

Systemic Lupus Erythematosus and Granulomatous Lymphadenopathy
Shrestha et al. BMC Pediatrics 2013, 13:179 http://www.biomedcentral.com/1471-2431/13/179 CASE REPORT Open Access Systemic lupus erythematosus and granulomatous lymphadenopathy Devendra Shrestha1*, Ajaya Kumar Dhakal1, Shiva Raj KC2, Arati Shakya1, Subhash Chandra Shah1 and Henish Shakya1 Abstract Background: Systemic lupus erythematosus (SLE) is known to present with a wide variety of clinical manifestations. Lymphadenopathy is frequently observed in children with SLE and may occasionally be the presenting feature. SLE presenting with granulomatous changes in lymph node biopsy is rare. These features may also cause diagnostic confusion with other causes of granulomatous lymphadenopathy. Case presentation: We report 12 year-old female who presented with generalized lymphadenopathy associated with intermittent fever as well as weight loss for three years. She also had developed anasarca two years prior to presentation. On presentation, she had growth failure and delayed puberty. Lymph node biopsy revealed granulomatous features. She developed a malar rash, arthritis and positive ANA antibodies over the course of next two months and showed WHO class II lupus nephritis on renal biopsy, which confirmed the final diagnosis of SLE. She was started on oral prednisolone and hydroxychloroquine with which her clinical condition improved, and she is currently much better under regular follow up. Conclusion: Generalized lymphadenopathy may be the presenting feature of SLE and it may preceed the other symptoms of SLE by many years as illustrated by this patient. Granulomatous changes may rarely be seen in lupus lymphadenitis. Although uncommon, in children who present with generalized lymphadenopathy along with prolonged fever and constitutional symptoms, non-infectious causes like SLE should also be considered as a diagnostic possibility. -
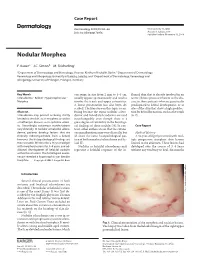
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable. -

Topical Photodynamic Therapy for Localized Scleroderma
Acta Derm Venereol 2000; 80: 26±27 Topical Photodynamic Therapy for Localized Scleroderma SIGRID KARRER, CHRISTOPH ABELS, MICHAEL LANDTHALER and ROLF-MARKUS SZEIMIES Department of Dermatology, University of Regensburg, Regensburg, Germany Therapy of localized scleroderma is unsatisfactory, with MATERIAL AND METHODS numerous treatments being used that have only limited success Patients or considerable side-effects. The aim of this trial was to determine whether topical photodynamic therapy would be Five patients aged between 21 and 63 years with biopsy-proven effective in patients with localized scleroderma. Five patients localized scleroderma (morphoea) were studied. All patients had evidence of progressive disease, i.e. lesions showed an in¯ammatory with progressive disease, in whom conventional therapies had reaction and increase in size. In each patient the condition had failed, were treated by application of a gel containing 3% previously failed to respond to potent topical corticosteroids, systemic 5-aminolevulinic acid followed by irradiation with an incoherent therapy with penicillin and/or PUVA bath photochemotherapy. lamp (40 mW/cm2, 10 J/cm2). The treatment was performed Patients were required to discontinue all therapy at least 4 weeks once or twice weekly for 3 ± 6 months. In all patients the before study initiation. The most affected sclerotic plaque of each therapy was highly effective for sclerotic plaques, as measured patient was judged at baseline and then every 2 weeks during by a quantitative durometer score and a clinical skin score. The treatment. Sclerotic plaques were assessed by a clinical skin score (10) only side-effect was a transient hyperpigmentation of the and the durometer score (11). -

Tumid Lupus Erythematosus
Tumid Lupus Erythematosus Sylvia Hsu, MD; Linda Y. Hwang, MD; Hiram Ruiz, MD Tumid lupus erythematosus (TLE) is a variant of cutaneous lupus erythematosus. Most patients who present with these skin lesions are young women. The condition clinically resembles polymorphous light eruption, systemic lupus ery- thematosus (SLE), reticulated erythematous mucinosis, or gyrate erythema. Histopathologi- cally, the lesions resemble classic lupus erythem- atosus because of their superficial and deep lymphohistiocytic inflammatory infiltrates and dermal mucin. However, unlike classic lupus ery- thematosus, there is little or no epidermal or dermo-epidermal involvement. Antinuclear anti- body test results are usually negative. We describe 4 cases of TLE and discuss the differ- ential diagnosis. here have been few reports of tumid lupus erythematosus (TLE) in the literature.1-4 T Most major textbooks of dermatology or Figure 1. Erythematous papules and plaques on the dermatopathology mention this entity only briefly, chest of patient 1. if at all. Ackerman et al5 consider TLE to be a manifestation of discoid lupus erythematosus (DLE). We describe 4 patients with TLE and review the her chest, upper extremities, and face (Figure 1). list of controversial entities that overlap clinically Test results for antinuclear antibodies (ANA), Ro, and histologically with TLE. La, and dsDNA were all negative. Histopathology results revealed superficial and deep perivascular Case Reports and periadnexal lymphohistiocytic infiltrates with Patient 1—A 34-year-old white woman presented dermal mucin. There was no involvement of the with an 8-year history of asymptomatic lesions on epidermis, the dermo-epidermal junction, or the her face, neck, chest, and upper extremities. -

Clinical Spectrum of Lyme Disease
European Journal of Clinical Microbiology & Infectious Diseases (2019) 38:201–208 https://doi.org/10.1007/s10096-018-3417-1 REVIEW Clinical spectrum of Lyme disease Jesus Alberto Cardenas-de la Garza1 & Estephania De la Cruz-Valadez1 & Jorge Ocampo-Candiani 1 & Oliverio Welsh1 Received: 4 September 2018 /Accepted: 30 October 2018 /Published online: 19 November 2018 # Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Lyme disease (borreliosis) is one of the most common vector-borne diseases worldwide. Its incidence and geographic expansion has been steadily increasing in the last decades. Lyme disease is caused by Borrelia burgdorferi sensu lato, a heterogeneous group of which three genospecies have been systematically associated to Lyme disease: B. burgdorferi sensu stricto Borrelia afzelii and Borrelia garinii. Geographical distribution and clinical manifestations vary according to the species involved. Lyme disease clinical manifestations may be divided into three stages. Early localized stage is characterized by erythema migrans in the tick bite site. Early disseminated stage may present multiple erythema migrans lesions, borrelial lymphocytoma, lyme neuroborreliosis, carditis, or arthritis. The late disseminated stage manifests with acordermatitis chronica atrophicans, lyme arthritis, and neurological symptoms. Diagnosis is challenging due to the varied clinical manifestations it may present and usually involves a two-step serological approach. In the current review, we present a thorough revision of the clinical manifestations Lyme disease may present. Additionally, history, microbiology, diagnosis, post-treatment Lyme disease syndrome, treatment, and prognosis are discussed. Keywords Lyme disease . Borrelia burgdorferi . Tick-borne diseases . Ixodes . Erythema migrans . Lyme neuroborreliosis History posteriorly meningitis, establishing a link between both mani- festations. -

Oral Health Care for Patients with Epidermolysis Bullosa
Oral Health Care for Patients with Epidermolysis Bullosa Best Clinical Practice Guidelines October 2011 Oral Health Care for Patients with Epidermolysis Bullosa Best Clinical Practice Guidelines October 2011 Clinical Editor: Susanne Krämer S. Methodological Editor: Julio Villanueva M. Authors: Prof. Dr. Susanne Krämer Dr. María Concepción Serrano Prof. Dr. Gisela Zillmann Dr. Pablo Gálvez Prof. Dr. Julio Villanueva Dr. Ignacio Araya Dr. Romina Brignardello-Petersen Dr. Alonso Carrasco-Labra Prof. Dr. Marco Cornejo Mr. Patricio Oliva Dr. Nicolás Yanine Patient representatives: Mr. John Dart Mr. Scott O’Sullivan Pilot: Dr. Victoria Clark Dr. Gabriela Scagnet Dr. Mariana Armada Dr. Adela Stepanska Dr. Renata Gaillyova Dr. Sylvia Stepanska Review: Prof. Dr. Tim Wright Dr. Marie Callen Dr. Carol Mason Prof. Dr. Stephen Porter Dr. Nina Skogedal Dr. Kari Storhaug Dr. Reinhard Schilke Dr. Anne W Lucky Ms. Lesley Haynes Ms. Lynne Hubbard Mr. Christian Fingerhuth Graphic design: Ms. Isabel López Production: Gráfica Metropolitana Funding: DEBRA UK © DEBRA International This work is subject to copyright. ISBN-978-956-9108-00-6 Versión On line: ISBN 978-956-9108-01-3 Printed in Chile in October 2011 Editorial: DEBRA Chile Acknowledgement: We would like to thank Coni V., María Elena, María José, Daniela, Annays, Lisette, Victor, Coni S., Esteban, Coni A., Felipe, Nibaldo, María, Cristián, Deyanira and Victoria for sharing their smile to make these Guidelines more friendly. 4 Contents 1 Introduction 07 2 Oral care for patients with Inherited Epidermolysis Bullosa 11 3 Dental treatment 19 4 Anaesthetic management 29 5 Summary of recommendations 33 Development of the guideline 37 6 Appendix 43 7.1 List of abbreviations and glossary 7.2 Oral manifestations of Epidermolysis Bullosa 7 7.3 General information on Epidermolysis Bullosa 7.4 Exercises for mouth, jaw and tongue 8 References 61 5 A message from the patient representative: “Be guided by the professionals. -

Immune Thrombocytopenic Purpura (ITP) — Adult Conditions for Which Ivig Has an Established Therapeutic Role
Immune thrombocytopenic purpura (ITP) — adult Conditions for which IVIg has an established therapeutic role. Specific Conditions Newly Diagnosed Immune thrombocytopenic purpura (ITP) Persistent Immune thrombocytopenic purpura (ITP) Chronic Immune thrombocytopenic purpura (ITP) Evans syndrome ‐ with significant Immune thrombocytopenic purpura (ITP) ‐ adult Indication for IVIg Use Newly diagnosed ITP — initial Ig therapy ITP in pregnancy — initial Ig therapy ITP with life‐threatening haemorrhage or the potential for life‐threatening haemorrhage Newly diagnosed or persistent ITP — subsequent therapy (diagnosis <12 months) Refractory persistent or chronic ITP — splenectomy failed or contraindicated and second‐line agent unsuccessful Subsequent or ongoing treatment for ITP responders during pregnancy and the postpartum period ITP and inadequate platelet count for planned surgery HIV‐associated ITP Level of Evidence Evidence of probable benefit – more research needed (Category 2a) Description and Diagnostic Immune thrombocytopenic purpura (ITP) is a reduction in platelet count Criteria (thrombocytopenia) resulting from shortened platelet survival due to anti‐platelet antibodies, reduced platelet production due to immune induced reduced megakaryopoeisis and/or immune mediated direct platelet lysis. When counts are very low (less than 30x109/L), bleeding into the skin (purpura) and mucous membranes can occur. Bone marrow platelet production (megakaryopoiesis) is morphologically normal. In some cases, there is additional impairment of platelet function related to antibody binding to glycoproteins on the platelet surface. It is a common finding in patients with human immunodeficiency virus (HIV) disease, and while it may be found at any stage of the infection, its prevalence increases as HIV disease advances. Around 80 percent of adults with ITP have the chronic form of disease. -

Systemic Sclerosis/Scleroderma: a Treatable Multisystem Disease
Systemic Sclerosis/Scleroderma: A Treatable Multisystem Disease MONIQUE HINCHCLIFF, MD, and JOHN VARGA, MD Northwestern University, Feinberg School of Medicine, Chicago, Illinois Systemic sclerosis (systemic scleroderma) is a chronic connective tissue disease of unknown etiology that causes wide- spread microvascular damage and excessive deposition of collagen in the skin and internal organs. Raynaud phenome- non and scleroderma (hardening of the skin) are hallmarks of the disease. The typical patient is a young or middle-age woman with a history of Raynaud phenomenon who presents with skin induration and internal organ dysfunction. Clinical evaluation and laboratory testing, along with pulmonary function testing, Doppler echocardiography, and high-resolution computed tomography of the chest, establish the diagnosis and detect visceral involvement. Patients with systemic sclerosis can be classified into two distinct clinical subsets with different patterns of skin and internal organ involvement, autoantibody production, and survival. Prognosis is determined by the degree of internal organ involvement. Although no disease-modifying therapy has been proven effective, complications of systemic sclerosis are treatable, and interventions for organ-specific manifestations have improved substantially. Medications (e.g., cal- cium channel blockers and angiotensin-II receptor blockers for Raynaud phenomenon, appropriate treatments for gastroesophageal reflux disease) and lifestyle modifications can help prevent complications, such as digital ulcers and Barrett esophagus. Endothelin-1 receptor blockers and phosphodiesterase-5 inhibitors improve pulmonary arte- rial hypertension. The risk of renal damage from scleroderma renal crisis can be lessened by early detection, prompt initiation of angiotensin-converting enzyme inhibitor therapy, and avoidance of high-dose corticosteroids. Optimal patient care includes an integrated, multidisciplinary approach to promptly and effectively recognize, evaluate, and manage complications and limit end-organ dysfunction.