Classics in Indian Medicine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glycobiology Protocols M E T H O D S I N M O L E C U L a R B I O L O G Y™
Glycobiology Protocols M E T H O D S I N M O L E C U L A R B I O L O G Y™ John M. Walker, SERIES EDITOR 384. Capillary Electrophoresis: Methods and Protocols, 357. Cardiovascular Proteomics: Methods and Protocols, edited by Philippe Schmitt-Kopplin, 2007 edited by Fernando Vivanco, 2006 383. Cancer Genomics and Proteomics: Methods and 356. High-Content Screening: A Powerful Approach Protocols, edited by Paul B. Fisher, 2007 to Systems Cell Biology and Drug Discovery, 382. Microarrays, Second Edition: Volume 2, Applications edited by D. Lansing Taylor, Jeffrey Haskins, and Data Analysis, edited by Jang B. Rampal, 2007 and Ken Guiliano, 2007 381. Microarrays, Second Edition: Volume 1, Synthesis 355. Plant Proteomics: Methods and Protocols, edited Methods, edited by Jang B. Rampal, 2007 by Hervé Thiellement, Michel Zivy, Catherine 380. Immunological Tolerance: Methods and Protocols, Damerval, and Valerie Mechin, 2006 edited by Paul J. Fairchild, 2007 354. Plant–Pathogen Interactions: Methods and 379. Glycovirology Protocols, edited by Richard J. Protocols, edited by Pamela C. Ronald, 2006 Sugrue, 2007 353. DNA Analysis by Nonradioactive Probes: Methods 378. Monoclonal Antibodies: Methods and Protocols, and Protocols, edited by Elena Hilario and John. F. edited by Maher Albitar, 2007 MacKay, 2006 377. Microarray Data Analysis: Methods and 3352.52 Protein Engineering Protocols, edited by Kristian Applications, edited by Michael J. Korenberg, 2007 Müller and Katja Arndt, 2006 376. Linkage Disequilibrium and Association 3351.51 C. elegans: Methods and Applications, edited by Mapping: Analysis and Application, edited by Kevin Strange, 2006 Andrew R. Collins, 2007 3350.50 Protein Folding Protocols, edited by Yawen Bai 375. -

CHEM 537: Carbohydrate Biochemistry and Glycobiology Instructor: Professor Anthony S
CHEM 537: Carbohydrate Biochemistry and Glycobiology Instructor: Professor Anthony S. Serianni Fall 2014 8:10-9:15 AM, MWF, 322 Jordan November 14 – December 12, 2014 PART A: Monosaccharides, Oligosaccharides and Polysaccharides Textbook Biochemistry, 4th Edition, Voet/Voet, Wiley, 2011 Chapter 11: Sugars and Polysaccharides Chapter 23: Other Pathways of Carbohydrate Metabolism Supplemental Text (useful for course; on reserve in Chem/Phys Library) M. E. Taylor and K. Drickamer, Introduction to Glycobiology, 3rd Ed., Oxford, 2011 Literature Reading: Distributed electronically Topics: Aldoses and ketoses: structures, nomenclature, absolute configuration Cyclization: furanose and pyranose ring forms; anomeric configuration Anomerization (implications for saccharide binding proteins) Relative stabilities of cyclic forms Acyclic forms: aldehydo and keto forms, and their hydrates Ring conformation: conformational averaging Exocyclic conformations (C-O, N-acetyl, CH2OH) Amphiphilic character of saccharides (implications for receptor binding) Saccharide solvation: H-bonding behaviors Monosaccharide derivatives: Phosphate esters Sulfate esters Aminosugars Deoxysugars Alditols Aldonic acids (lactones) Uronic acids Dicarbonyl sugars (osones) α-ketoacids (sialic acid, KDO) Aldose-ketose isomerization (chemical, biological) Di- and oligosaccharide nomenclature Formation of glycosidic bonds: disaccharides (chemical, biological)s Mechanisms of glycoside bond formation and hydrolysis Phi/psi plots for glycosidic linkages Factors affecting linkage conformation; -
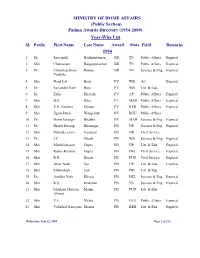
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Bimal Kumar Bachhawat
Bimal Kumar Bachhawat. (6 August 1925-23 September 1996) President, Neurological Society of India. 1973. Dr. Bachhawat did his M. Sc. from Calcutta University in 19,18 and obtained his doctoral degree from the University of Illinois, USA in 1953 under the supervision of a well-known enzymologist, Professor Vestling. Dr. Bachhawat returned to India in 1957 and joined the Christian Medical College and Hospital, Vellore where. he established the first neurochemistry laboratory in the country. ln 1976, he assumed the directorship of the Indian Institute of Chemical Biology, Calcutta and continued to lead this institute till 1985. He then moved to the University of Delhi South Campus (UDSC) as Professor and Head of the Department of Biochemistry and played a crucial role in the developntent of the Life Sciences departments. After his retirement in i990 he continued at the Deparmtnet of Biochemistry as the Bhatnagar Fellow of the Council of Scientific and industrial Research (CSIR) till 1995 and then took over the co- ordinatorship of the D. S. Kothari Centre for Science and Ethics at UDSC. His pioneering research contributions in the area of biochemical basis of genetic disorders, liposomes as model membranes and development of targeted drug delivery s),stems brought him widespread recognition. A Fellow of the Indian Academy of Sciences, National Academy of Sciences and Indian National Science Academy (I\SA), he served as President of the Society of Biological Chemists (India), the first Indian President of the Federation of Asian ana Oceanian Biochemistry and President of the NationaI Organizing Committe of the International Union of Biochemistry and Molecular Biology, 1994.He received a large number of awards and honours in his illustrious career including the Shanti Swarup Bhatnagar Prize in 1962, the Amrut Mody Research Award in 1974, the J. -

Glycomics Hits the Big Time
Leading Edge Essay Glycomics Hits the Big Time Gerald W. Hart1,* and Ronald J. Copeland1 1Department of Biological Chemistry, School of Medicine, Johns Hopkins University, 725 North Wolfe Street, Baltimore, MD 21205-2185, USA *Correspondence: [email protected] DOI 10.1016/j.cell.2010.11.008 Cells run on carbohydrates. Glycans, sequences of carbohydrates conjugated to proteins and lipids, are arguably the most abundant and structurally diverse class of molecules in nature. Recent advances in glycomics reveal the scope and scale of their functional roles and their impact on human disease. By analogy to the genome, transcriptome, O-GlcNAc (Hart et al., 2007). Even though Dynamic Structural Complexity or proteome, the ‘‘glycome’’ is the the generic term ‘‘glycosylation’’ is often Underlies Glycan Functions complete set of glycans and glycoconju- used to categorize and lump all glycan Glycoconjugates provide dynamic struc- gates that are made by a cell or organism modifications of proteins into one bin, tural diversity to proteins and lipids that under specific conditions. Therefore, side by side with other posttranslational is responsive to cellular phenotype, to ‘‘glycomics’’ refers to studies that attempt modifications such as phosphorylation, metabolic state, and to the developmental to define or quantify the glycome of a cell, acetylation, ubiquitination, or methylation, stage of cells. Complex glycans play crit- tissue, or organism (Bertozzi and Sasise- such a view is not only inaccurate, but ical roles in intercellular and intracellular kharan, 2009). In eukaryotes, protein also is completely misleading. If one only processes, which are fundamentally glycosylation generally involves the cova- considers the linkage of the first glycan important to the development of multicel- lent attachment of glycans to serine, to the polypeptide in both prokaryotic lularity (Figure 1). -

MANALI PETROCHEMICALS LIMITED CIN : L24294TN1986PLC013087 Regd Off: 'SPIC HOUSE', 88, Mount Road, Guindy, Chennai- 600 032
MANALI PETROCHEMICALS LIMITED CIN : L24294TN1986PLC013087 Regd Off: 'SPIC HOUSE', 88, Mount Road, Guindy, Chennai- 600 032. Tele-Fax No.: 044-22351098 Email: [email protected], Website: www.manalipetro.com DETAILS OF SHARES TO BE TRANSFERRED TO INVESTOR EDUCATION & PROTECTION FUND ON WHICH NO DIVIDEND HAS BEEN CLAIMED FOR THE FY 2008-09 TO 2015-16 SL.NO FOLIO_DP_ID_CL_ID NAME OF THE SHAREHOLDER NO.OF.SHARES TOBE TRFD TO IEPF 1 A0000033 SITARAMAN G 450 2 A0000089 LAKSHMANAN CHELLADURAI 300 3 A0000093 MANI N V S 150 4 A0000101 KUNNATH NARAYANAN SUBRAMANIAN 300 5 A0000120 GOPAL THACHAT MURALIDHAR 300 6 A0000130 ROY FESTUS 150 7 A0000140 SATHYAMURTHY N 300 8 A0000142 MOHAN RAO V 150 9 A0000170 MURALIDHARAN M R 300 10 A0000171 CHANDRASEKAR V 150 11 A0000187 VISWANATH J 300 12 A0000191 JAGMOHAN SINGH BIST 300 13 A0000213 MURUGANANDAN RAMACHANDRAN 150 14 A0000219 SHANMUGAM E 600 15 A0000232 VENKATRAMAN N 150 16 A0000235 KHADER HUSSAINY S M 150 17 A0000325 PARAMJEET SINGH BINDRA 300 18 A0000332 SELVARAJU G 300 19 A0000334 RAJA VAIDYANATHAN R 300 20 A0000339 PONNUSWAMY SAMPANGIRAM 300 21 A0000356 GANESH MAHADHEVAN 150 22 A0000381 MEENAKSHI SUNDARAM K 150 23 A0000389 CHINNIAH A 150 24 A0000392 PERUMAL K 300 25 A0000423 CHANDRASEKARAN C 300 26 A0000450 RAMAMOORTHY NAIDU MADUPURI 150 27 A0000473 ZULFIKAR ALI SULTAN MOHAMMAD 300 28 A0000550 SRINIVASAN K 150 29 A0000556 KANAKAMUTHU A 300 30 A0000561 KODANDA PANI CHIVUKULA 300 31 A0000565 VARADHAN R 150 32 A0000566 KARTHIGEYAN S 150 33 A0000598 RAMASASTRULU TRIPIRNENI 150 34 A0000620 -

Prof Benjamin G. Davis
Prof Benjamin G. Davis Professor of Chemistry, Fellow and Tutor in Organic Chemistry, Pembroke College Ben Davis got his B.A. (1993) and D.Phil. (1996) from the University of Oxford. During this time he learnt the beauty of carbohydrate chemistry under the supervision of Professor George Fleet. He then spent 2 years as a postdoctoral fellow in the laboratory of Professor Bryan Jones at the University of Toronto, exploring protein chemistry and biocatalysis. In 1998 he returned to the U.K. to take up a lectureship at the University of Durham. In the autumn of 2001 he moved to the Dyson Perrins Laboratory, University of Oxford and received a fellowship at Pembroke College, Oxford. He was promoted to Full Professor in 2005. His group's research centres on the chemical understanding and exploitation of biomolecular function (Synthetic Biology, Chemical Biology and Chemical Medicine), with an emphasis on carbohydrates and proteins. In particular, the group's interests encompass synthesis and methodology; target biomolecule synthesis; inhibitor/probe/substrate design; biocatalysis; enzyme & biomolecule mechanism; biosynthetic pathway determination; protein engineering; drug delivery; molecular biology; structural biology; cell biology; glycobiology; molecular imaging and in vivo biology. This work has been recognised by Prizes and Awards and named Lecturerships. He sits (has sat) on the Editorial / Editorial Advisory Boards of Carbohydrate Research (2005-2012), Chemical Biology and Drug Design (2006-), Organic and Biomolecular Chemistry (2006-2011), the Biochemical Journal (Advisory Board 2002-2005, Editorial Board 2009-2016), Chemical Science (2010-2012, 2015-) and ChemBioChem (2011-). He was the Editor-in-Chief of Bioorganic Chemistry (2011-2013) and an Associate Editor of Chemical Science (2012-14). -
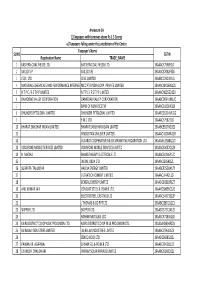
FINAL DISTRIBUTION.Xlsx
Annexure-1A 1)Taxpayers with turnover above Rs 1.5 Crores a) Taxpayers falling under the jurisdiction of the Centre Taxpayer's Name SL NO GSTIN Registration Name TRADE_NAME 1 EASTERN COAL FIELDS LTD. EASTERN COAL FIELDS LTD. 19AAACE7590E1ZI 2 SAIL (D.S.P) SAIL (D.S.P) 19AAACS7062F6Z6 3 CESC LTD. CESC LIMITED 19AABCC2903N1ZL 4 MATERIALS CHEMICALS AND PERFORMANCE INTERMEDIARIESMCC PTA PRIVATE INDIA CORP.LIMITED PRIVATE LIMITED 19AAACM9169K1ZU 5 N T P C / F S T P P LIMITED N T P C / F S T P P LIMITED 19AAACN0255D1ZV 6 DAMODAR VALLEY CORPORATION DAMODAR VALLEY CORPORATION 19AABCD0541M1ZO 7 BANK OF NOVA SCOTIA 19AAACB1536H1ZX 8 DHUNSERI PETGLOBAL LIMITED DHUNSERI PETGLOBAL LIMITED 19AAFCD5214M1ZG 9 E M C LTD 19AAACE7582J1Z7 10 BHARAT SANCHAR NIGAM LIMITED BHARAT SANCHAR NIGAM LIMITED 19AABCB5576G3ZG 11 HINDUSTAN UNILEVER LIMITED 19AAACH1004N1ZR 12 GUJARAT COOPERATIVE MILKS MARKETING FEDARATION LTD 19AAAAG5588Q1ZT 13 VODAFONE MOBILE SERVICES LIMITED VODAFONE MOBILE SERVICES LIMITED 19AAACS4457Q1ZN 14 N MADHU BHARAT HEAVY ELECTRICALS LTD 19AAACB4146P1ZC 15 JINDAL INDIA LTD 19AAACJ2054J1ZL 16 SUBRATA TALUKDAR HALDIA ENERGY LIMITED 19AABCR2530A1ZY 17 ULTRATECH CEMENT LIMITED 19AAACL6442L1Z7 18 BENGAL ENERGY LIMITED 19AADCB1581F1ZT 19 ANIL KUMAR JAIN CONCAST STEEL & POWER LTD.. 19AAHCS8656C1Z0 20 ELECTROSTEEL CASTINGS LTD 19AAACE4975B1ZP 21 J THOMAS & CO PVT LTD 19AABCJ2851Q1Z1 22 SKIPPER LTD. SKIPPER LTD. 19AADCS7272A1ZE 23 RASHMI METALIKS LTD 19AACCR7183E1Z6 24 KAIRA DISTRICT CO-OP MILK PRO.UNION LTD. KAIRA DISTRICT CO-OP MILK PRO.UNION LTD. 19AAAAK8694F2Z6 25 JAI BALAJI INDUSTRIES LIMITED JAI BALAJI INDUSTRIES LIMITED 19AAACJ7961J1Z3 26 SENCO GOLD LTD. 19AADCS6985J1ZL 27 PAWAN KR. AGARWAL SHYAM SEL & POWER LTD. 19AAECS9421J1ZZ 28 GYANESH CHAUDHARY VIKRAM SOLAR PRIVATE LIMITED 19AABCI5168D1ZL 29 KARUNA MANAGEMENT SERVICES LIMITED 19AABCK1666L1Z7 30 SHIVANANDAN TOSHNIWAL AMBUJA CEMENTS LIMITED 19AAACG0569P1Z4 31 SHALIMAR HATCHERIES LIMITED SHALIMAR HATCHERIES LTD 19AADCS6537J1ZX 32 FIDDLE IRON & STEEL PVT. -

SYNTHESIS Template V2.0
Journal of Chemical Reviews, 2020, Volume 2, Issue 1, Pages 1-27 Review Article A Review on Synthesis and Applications of Statin Family Drugs as a New Generations of Anti-Blood Medicines Sajedeh Safapoor a, Hamid Yazdani b,*, Parisa Shahabi c a Department of Chemistry, Iran University of Science and Technology, Tehran, Iran b Department of Chemical Engineering, Payame Noor University, Tehran, Iran c Department of Physical Chemistry , Alzahra University, Tehran, Iran Receive Date: 28 July 2019, Revise Date: 15 September 2019, Accept Date: 15 October2019 Abstract: Cardiovascular disease (CVD) represents one of the most important health problems. One of the main risk factors for CVD death is the high cholesterol levels. Statins have been shown to lower low-density lipoprotein (LDL) cholesterol levels and the risk of cardiovascular disease, and are currently the first line of treatment for hypercholesterolemia. Atorvastatin is one of the most effective drugs and is part of the blood lipid lowering drug group. This work studies the mechanism of the use of statin family drugs and atorvastatin to determine the clinical requirements for improving the dermatology and the statins. DOI: 10.33945/SAMI/JCR.2020.1.1 Keywords: Atorvastatin, High cholesterol, Cardiovascular disease, Statins, Blood lipid lowering effect Graphical Abstract: Biography: Sajedeh Safapoor was born in Iran, (Mazandaran), in 1994. She completed her BSc (2017) degree from University of Mazandaran in pure chemistry. She is at the end of her Master’s Degree in Organic Chemistry at the University of Science and Technology where she is working on the synthesis of nanomaterials for biological & catalysis application. -
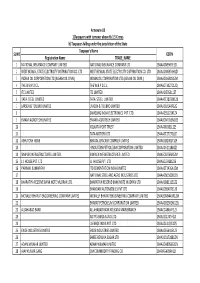
FINAL DISTRIBUTION.Xlsx
Annexure-1B 1)Taxpayers with turnover above Rs 1.5 Crores b) Taxpayers falling under the jurisdiction of the State Taxpayer's Name SL NO GSTIN Registration Name TRADE_NAME 1 NATIONAL INSURANCE COMPANY LIMITED NATIONAL INSURANCE COMPANY LTD 19AAACN9967E1Z0 2 WEST BENGAL STATE ELECTRICITY DISTRIBUTION CO. LTD WEST BENGAL STATE ELECTRICITY DISTRIBUTION CO. LTD 19AAACW6953H1ZX 3 INDIAN OIL CORPORATION LTD.(ASSAM OIL DIVN.) INDIAN OIL CORPORATION LTD.(ASSAM OIL DIVN.) 19AAACI1681G1ZM 4 THE W.B.P.D.C.L. THE W.B.P.D.C.L. 19AABCT3027C1ZQ 5 ITC LIMITED ITC LIMITED 19AAACI5950L1Z7 6 TATA STEEL LIMITED TATA STEEL LIMITED 19AAACT2803M1Z8 7 LARSEN & TOUBRO LIMITED LARSEN & TOUBRO LIMITED 19AAACL0140P1ZG 8 SAMSUNG INDIA ELECTRONICS PVT. LTD. 19AAACS5123K1ZA 9 EMAMI AGROTECH LIMITED EMAMI AGROTECH LIMITED 19AABCN7953M1ZS 10 KOLKATA PORT TRUST 19AAAJK0361L1Z3 11 TATA MOTORS LTD 19AAACT2727Q1ZT 12 ASHUTOSH BOSE BENGAL CRACKER COMPLEX LIMITED 19AAGCB2001F1Z9 13 HINDUSTAN PETROLEUM CORPORATION LIMITED. 19AAACH1118B1Z9 14 SIMPLEX INFRASTRUCTURES LIMITED. SIMPLEX INFRASTRUCTURES LIMITED. 19AAECS0765R1ZM 15 J.J. HOUSE PVT. LTD J.J. HOUSE PVT. LTD 19AABCJ5928J2Z6 16 PARIMAL KUMAR RAY ITD CEMENTATION INDIA LIMITED 19AAACT1426A1ZW 17 NATIONAL STEEL AND AGRO INDUSTRIES LTD 19AAACN1500B1Z9 18 BHARATIYA RESERVE BANK NOTE MUDRAN LTD. BHARATIYA RESERVE BANK NOTE MUDRAN LTD. 19AAACB8111E1Z2 19 BHANDARI AUTOMOBILES PVT LTD 19AABCB5407E1Z0 20 MCNALLY BHARAT ENGGINEERING COMPANY LIMITED MCNALLY BHARAT ENGGINEERING COMPANY LIMITED 19AABCM9443R1ZM 21 BHARAT PETROLEUM CORPORATION LIMITED 19AAACB2902M1ZQ 22 ALLAHABAD BANK ALLAHABAD BANK KOLKATA MAIN BRANCH 19AACCA8464F1ZJ 23 ADITYA BIRLA NUVO LTD. 19AAACI1747H1ZL 24 LAFARGE INDIA PVT. LTD. 19AAACL4159L1Z5 25 EXIDE INDUSTRIES LIMITED EXIDE INDUSTRIES LIMITED 19AAACE6641E1ZS 26 SHREE RENUKA SUGAR LTD. 19AADCS1728B1ZN 27 ADANI WILMAR LIMITED ADANI WILMAR LIMITED 19AABCA8056G1ZM 28 AJAY KUMAR GARG OM COMMODITY TRADING CO. -

CIN/BCIN Company/Bank Name
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e‐form IEPF‐2 CIN/BCIN L72200TG1990PLC011146 Prefill Company/Bank Name NCC LIMITED Date Of AGM(DD‐MON‐YYYY) 10‐AUG‐2018 Sum of unpaid and unclaimed dividend 412766.10 Sum of interest on matured debentures 0.00 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id‐Client Id‐ Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD‐MON‐YYYY) RENDUCHINTALA LAKSHMI NARASAMMA R VENKATRAMAIAH 5, ODULAPPA GARDENS, 1ST CROSS INDIA TELANGANA HYDERABAD P0000324 Amount for unclaimed and un 3000.00 01‐OCT‐2019 RAVINDER BATRA R C BATRA B‐124 AMAR COLONY LAJPAT NAGA INDIA DELHI NEW DELHI 110024 P0027254 Amount for unclaimed and un 3300.00 01‐OCT‐2019 LGIM BERMUDALTDACLGINDIAFUNDLTD NA C\O THE HONGKONG & SHANGHAI BINDIA MAHARASHTRA MUMBAI 400025 P0023027 Amount for unclaimed and un 2700.00 01‐OCT‐2019 ANIL SHARMA S C SHARMA C/O 33 RAGHAV RATNA TOWERS CHINDIA TELANGANA HYDERABAD -

D'alessio and Nancy M. Dahms Stigliano, Armando J. Parodi
Glycobiology and Extracellular Matrices: Structure of the Lectin Mannose 6-Phosphate Receptor Homology (MRH) Domain of Glucosidase II, an Enzyme That Regulates Glycoprotein Folding Quality Control in the Endoplasmic Reticulum Linda J. Olson, Ramiro Orsi, Solana G. Alculumbre, Francis C. Peterson, Ivan D. Stigliano, Armando J. Parodi, Cecilia Downloaded from D'Alessio and Nancy M. Dahms J. Biol. Chem. 2013, 288:16460-16475. doi: 10.1074/jbc.M113.450239 originally published online April 22, 2013 http://www.jbc.org/ Access the most updated version of this article at doi: 10.1074/jbc.M113.450239 Find articles, minireviews, Reflections and Classics on similar topics on the JBC Affinity Sites. by Luis Ielpi on November 14, 2013 Alerts: • When this article is cited • When a correction for this article is posted Click here to choose from all of JBC's e-mail alerts This article cites 51 references, 20 of which can be accessed free at http://www.jbc.org/content/288/23/16460.full.html#ref-list-1 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 288, NO. 23, pp. 16460–16475, June 7, 2013 © 2013 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. Structure of the Lectin Mannose 6-Phosphate Receptor Homology (MRH) Domain of Glucosidase II, an Enzyme That Regulates Glycoprotein Folding Quality Control in the Endoplasmic Reticulum* Received for publication, January 3, 2013, and in revised form, April 5, 2013 Published, JBC Papers in Press, April 22, 2013, DOI 10.1074/jbc.M113.450239 Linda J. Olson‡, Ramiro Orsi§, Solana G. Alculumbre§, Francis C.