Identification of a Metallopeptidase with TOP-Like Activity in Paracoccidioides Brasiliensis, with Increased Expression in A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002
USOO6395889B1 (12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002 (54) NUCLEIC ACID MOLECULES ENCODING WO WO-98/56804 A1 * 12/1998 ........... CO7H/21/02 HUMAN PROTEASE HOMOLOGS WO WO-99/0785.0 A1 * 2/1999 ... C12N/15/12 WO WO-99/37660 A1 * 7/1999 ........... CO7H/21/04 (75) Inventor: fish E. Robison, Wilmington, MA OTHER PUBLICATIONS Vazquez, F., et al., 1999, “METH-1, a human ortholog of (73) Assignee: Millennium Pharmaceuticals, Inc., ADAMTS-1, and METH-2 are members of a new family of Cambridge, MA (US) proteins with angio-inhibitory activity', The Journal of c: - 0 Biological Chemistry, vol. 274, No. 33, pp. 23349–23357.* (*) Notice: Subject to any disclaimer, the term of this Descriptors of Protease Classes in Prosite and Pfam Data patent is extended or adjusted under 35 bases. U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 09/392, 184 Primary Examiner Ponnathapu Achutamurthy (22) Filed: Sep. 9, 1999 ASSistant Examiner William W. Moore (51) Int. Cl." C12N 15/57; C12N 15/12; (74) Attorney, Agent, or Firm-Alston & Bird LLP C12N 9/64; C12N 15/79 (57) ABSTRACT (52) U.S. Cl. .................... 536/23.2; 536/23.5; 435/69.1; 435/252.3; 435/320.1 The invention relates to polynucleotides encoding newly (58) Field of Search ............................... 536,232,235. identified protease homologs. The invention also relates to 435/6, 226, 69.1, 252.3 the proteases. The invention further relates to methods using s s s/ - - -us the protease polypeptides and polynucleotides as a target for (56) References Cited diagnosis and treatment in protease-mediated disorders. -

Cloning and Expression of Leishmanolysin Gene from Leishmania Major in Primate Cell Lines
J. Sci. I. R. Iran Vol. 12, No. 2, Spring 2001 CLONING AND EXPRESSION OF LEISHMANOLYSIN GENE FROM LEISHMANIA MAJOR IN PRIMATE CELL LINES M. R. Razavi-Deligani1, M. Reza Sadaie2, V. Richinsky3, M. R. Noori- Daloii4, M. Azizi5, A. Amanzadeh6 and M. Assmar1* 1 Department of Parasitology, Pasteur Institute of Iran, Tehran, Islamic Republic of Iran 2 NovoMed Pharmaceuticals, Inc., Germantown, Maryland 20875, USA 3 Engelhardt Institute of Molecular Biology, Russian Academy of Science, Moscow, Russia 4 Department of Medical Genetic, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran 5 Department of Biotechnology, Pasteur Institute of Iran, Tehran, Islamic Republic of Iran 6 National Cell Bank, Pasteur Institute of Iran, Tehran, Islamic Republic of Iran Abstract Leishmanolysin is a worldwide disease that is caused by different species of the genus Leishmania. Leishmanolysin, One of the genes expressed by Leishmania, appears to be an ideal candidate for genetic vaccination. In this study, a full length sequence, which encodes Leishmanolysin functionally critical regions (amino acids 100-579), was cloned from a Leishmania strain endemic to Iran. Analysis by restriction enzyme digestion and DNA sequencing in pUC 19 based T-Vector showed that the cloned gene contained the conserved segments of the Leishmanolysin. The identified segments in predicted protein sequence of our clone contained the important domains that have been known to function at the attachment and internalization steps of the parasite life cycle. The cloned gene was expressed in human transformed muscle (Rhabdomyosarcoma TE671/RD) and African green monkey epithelial (COS-7) cell lines under cytomegalovirus (CMV) promoter, and the expressed protein was detected by enzyme linked immunosorbent assay. -

1 Metabolic Dysfunction Is Restricted to the Sciatic Nerve in Experimental
Page 1 of 255 Diabetes Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy Oliver J. Freeman1,2, Richard D. Unwin2,3, Andrew W. Dowsey2,3, Paul Begley2,3, Sumia Ali1, Katherine A. Hollywood2,3, Nitin Rustogi2,3, Rasmus S. Petersen1, Warwick B. Dunn2,3†, Garth J.S. Cooper2,3,4,5* & Natalie J. Gardiner1* 1 Faculty of Life Sciences, University of Manchester, UK 2 Centre for Advanced Discovery and Experimental Therapeutics (CADET), Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK 3 Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, UK 4 School of Biological Sciences, University of Auckland, New Zealand 5 Department of Pharmacology, Medical Sciences Division, University of Oxford, UK † Present address: School of Biosciences, University of Birmingham, UK *Joint corresponding authors: Natalie J. Gardiner and Garth J.S. Cooper Email: [email protected]; [email protected] Address: University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, United Kingdom Telephone: +44 161 275 5768; +44 161 701 0240 Word count: 4,490 Number of tables: 1, Number of figures: 6 Running title: Metabolic dysfunction in diabetic neuropathy 1 Diabetes Publish Ahead of Print, published online October 15, 2015 Diabetes Page 2 of 255 Abstract High glucose levels in the peripheral nervous system (PNS) have been implicated in the pathogenesis of diabetic neuropathy (DN). However our understanding of the molecular mechanisms which cause the marked distal pathology is incomplete. Here we performed a comprehensive, system-wide analysis of the PNS of a rodent model of DN. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

R&D Assay for Alzheimer's Disease
R&DR&D assayassay forfor Alzheimer’sAlzheimer’s diseasedisease Target screening⳼ Ⲽ㬔 antibody array, ᢜ⭉㬔 ⸽ἐⴐ Amyloid β-peptide Alzheimer’s disease⯸ ኸᷠ᧔ ᆹ⸽ inhibitor, antibody, ELISA kit Surwhrph#Surilohu#Dqwlerg|#Duud| 6OUSFBUFE 1."5SFBUFE )41 $3&# &3, &3, )41 $3&# &3, &3, 壤伡庰䋸TBNQMF ɅH 侴䋸嵄䍴䋸BOBMZUFT䋸䬱娴哜塵 1$ 1$ 1$ 1$ 5IFNPTUSFGFSFODFEBSSBZT 1$ 1$ QQ α 34, .4, 503 Q α 34, .4, 503 %SVHTDSFFOJOH0òUBSHFUFòFDUT0ATHWAY涭廐 6OUSFBUFE 堄币䋸4BNQMF侴䋸8FTUFSOPS&-*4"䍘䧽 1."5SFBUFE P 8FTUFSOCMPU廽喜儤应侴䋸0, Z 4VCTUSBUF -JHIU )31DPOKVHBUFE1BO "OUJQIPTQIPUZSPTJOF .FBO1JYFM%FOTJUZ Y $BQUVSF"OUJCPEZ 5BSHFU"OBMZUF "SSBZ.FNCSBOF $3&# &3, &3, )41 .4, Q α 34, 503 Human XL Cytokine Array kit (ARY022, 102 analytes) Adiponectin,Aggrecan,Angiogenin,Angiopoietin-1,Angiopoietin-2,BAFF,BDNF,Complement,Component C5/C5a,CD14,CD30,CD40L, Chitinase 3-like 1,Complement Factor D,C-Reactive Protein,Cripto-1,Cystatin C,Dkk-1,DPPIV,EGF,EMMPRIN,ENA-78,Endoglin, Fas L,FGF basic,FGF- 7,FGF-19,Flt-3 L,G-CSF,GDF-15,GM-CSF,GRO-α,Grow th Hormone,HGF,ICAM-1,IFN-γ,IGFBP-2,IGFBP-3, IL-1α,IL-1β, IL-1ra,IL-2,IL-3,IL-4,IL- 5,IL-6,IL-8, IL-10,IL-11,IL-12, IL-13,IL-15,IL-16,IL-17A,IL-18 BPa,IL-19,IL-22, IL-23,IL-24,IL-27, IL-31,IL-32α/β/γ,IL-33,IL-34,IP-10,I-TAC,Kallikrein 3,Leptin,LIF,Lipocalin-2,MCP-1,MCP-3,M-CSF,MIF,MIG,MIP-1α/MIP-1β,MIP-3α,MIP-3β,MMP-9, Myeloperoxidase,Osteopontin, p70, PDGF-AA, PDGF-AB/BB,Pentraxin-3, PF4, RAGE, RANTES,RBP4,Relaxin-2, Resistin,SDF-1α,Serpin E1, SHBG, ST2, TARC,TFF3,TfR,TGF- ,Thrombospondin-1,TNF-α, uPAR, VEGF, Vitamin D BP Human Protease (34 analytes) / -

Mitochondrial Protein Quality Control Mechanisms
G C A T T A C G G C A T genes Review Mitochondrial Protein Quality Control Mechanisms Pooja Jadiya * and Dhanendra Tomar * Center for Translational Medicine, Lewis Katz School of Medicine, Temple University, Philadelphia, PA 19140, USA * Correspondence: [email protected] (P.J.); [email protected] (D.T.); Tel.: +1-215-707-9144 (D.T.) Received: 29 April 2020; Accepted: 15 May 2020; Published: 18 May 2020 Abstract: Mitochondria serve as a hub for many cellular processes, including bioenergetics, metabolism, cellular signaling, redox balance, calcium homeostasis, and cell death. The mitochondrial proteome includes over a thousand proteins, encoded by both the mitochondrial and nuclear genomes. The majority (~99%) of proteins are nuclear encoded that are synthesized in the cytosol and subsequently imported into the mitochondria. Within the mitochondria, polypeptides fold and assemble into their native functional form. Mitochondria health and integrity depend on correct protein import, folding, and regulated turnover termed as mitochondrial protein quality control (MPQC). Failure to maintain these processes can cause mitochondrial dysfunction that leads to various pathophysiological outcomes and the commencement of diseases. Here, we summarize the current knowledge about the role of different MPQC regulatory systems such as mitochondrial chaperones, proteases, the ubiquitin-proteasome system, mitochondrial unfolded protein response, mitophagy, and mitochondria-derived vesicles in the maintenance of mitochondrial proteome and health. The proper understanding of mitochondrial protein quality control mechanisms will provide relevant insights to treat multiple human diseases. Keywords: mitochondria; proteome; ubiquitin; proteasome; chaperones; protease; mitophagy; mitochondrial protein quality control; mitochondria-associated degradation; mitochondrial unfolded protein response 1. Introduction Mitochondria are double membrane, dynamic, and semiautonomous organelles which have several critical cellular functions. -

Structure of Neurolysin Reveals a Deep Channel That Limits Substrate Access
Structure of neurolysin reveals a deep channel that limits substrate access C. Kent Brown*†, Kevin Madauss*, Wei Lian‡, Moriah R. Beck§, W. David Tolbert¶, and David W. Rodgersʈ Department of Molecular and Cellular Biochemistry and Center for Structural Biology, University of Kentucky, Lexington, KY 40536 Communicated by Stephen C. Harrison, Harvard University, Cambridge, MA, December 29, 2000 (received for review November 14, 2000) The zinc metallopeptidase neurolysin is shown by x-ray crystallog- cytosolic, but it also can be secreted or associated with the raphy to have large structural elements erected over the active site plasma membrane (11), and some of the enzyme is made with a region that allow substrate access only through a deep narrow mitochondrial targeting sequence by initiation at an alternative channel. This architecture accounts for specialization of this neu- transcription start site (12). ropeptidase to small bioactive peptide substrates without bulky Although neurolysin cleaves a number of neuropeptides in secondary and tertiary structures. In addition, modeling studies vitro, its most established (5, 13, 14) role in vivo (along with indicate that the length of a substrate N-terminal to the site of thimet oligopeptidase) is in metabolism of neurotensin, a 13- hydrolysis is restricted to approximately 10 residues by the limited residue neuropeptide. It hydrolyzes this peptide between resi- size of the active site cavity. Some structural elements of neuro- dues 10 and 11, creating shorter fragments that are believed to  lysin, including a five-stranded -sheet and the two active site be inactive. helices, are conserved with other metallopeptidases. The connect- Neurotensin (pGlu-Leu-Tyr-Gln-Asn-Lys-Pro-Arg-Arg- ing loop regions of these elements, however, are much extended Pro s Tyr-Ile-Leu) is found in a variety of peripheral and in neurolysin, and they, together with other open coil elements, central tissues where it is involved in a number of effects, line the active site cavity. -
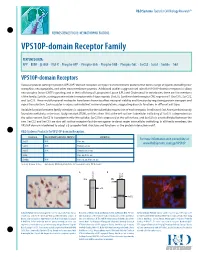
VPS10P-Domain Receptor Family
R&D Systems Tools for Cell Biology Research™ NEUROSCIENCE FOCUS: NEUROTROPHIC FACTORS VPS10P-domain Receptor Family FEATURED DATA: APP · BDNF · b-NGF · NGF R · Phospho-APP · Phospho-TrkA · Phospho-TrkB · Phospho-TrkC · SorCS2 · SorLA · Sortilin · TrkA VPS10P-domain Receptors Vacuolar protein sorting 10 protein (VPS10P)-domain receptors are type I transmembrane proteins that bind a range of ligands including neu- rotrophins, neuropeptides, and other transmembrane proteins. Additional studies suggest novel roles for VPS10P-domain receptors in ciliary neurotrophic factor (CNTF) signaling, and in the trafficking of Lipoprotein Lipase (LPL) and Cholesterol. In vertebrates, there are five members of the family, Sortilin, sorting protein-related receptor with A-type repeats (SorLA), Sortilin-related receptor CNS expressed 1 (SorCS1), SorCS2, and SorCS3. These multifunctional molecules have been shown to affect neuronal viability and function by regulating protein transport and signal transduction. Each receptor is expressed in distinct neuronal populations, suggesting discrete functions in different cell types. Variable function between family members is supported by the subcellular expression of each receptor. Sortilin and SorLA are predominantly found intracellularly, in the trans-Golgi network (TGN), with less than 10% at the cell surface. Subcellular trafficking of SorCS1 is dependent on the splice variant. SorCS1a is predominantly intracellular, SorCS1b is expressed at the cell surface, and SorCS1c is evenly divided between the two. SorCS2 -

Stress-Triggered Activation of the Metalloprotease Oma1 Involves Its
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Biochemistry -- Faculty Publications Biochemistry, Department of 2014 Stress-triggered Activation of the Metalloprotease Oma1 Involves Its C-terminal Region and Is Important for Mitochondrial Stress Protection in Yeast Iryna Bohovych University of Nebraska- Lincoln, [email protected] Garrett onD aldson University of Nebraska- Lincoln Sara Christianson University of Nebraska-Lincoln Nataliya Zahayko University of Nebraska-Lincoln Oleh Khalimonchuk University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/biochemfacpub Part of the Biochemistry Commons, Biotechnology Commons, and the Other Biochemistry, Biophysics, and Structural Biology Commons Bohovych, Iryna; Donaldson, Garrett; Christianson, Sara; Zahayko, Nataliya; and Khalimonchuk, Oleh, "Stress-triggered Activation of the Metalloprotease Oma1 Involves Its C-terminal Region and Is Important for Mitochondrial Stress Protection in Yeast" (2014). Biochemistry -- Faculty Publications. 167. http://digitalcommons.unl.edu/biochemfacpub/167 This Article is brought to you for free and open access by the Biochemistry, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Biochemistry -- Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 289, NO. 19, pp. 13259–13272, May 9, 2014 © 2014 by The American Society -

Next Generation Sequencing Identifies Five Major Classes of Potentially Therapeutic Enzymes Secreted by Lucilia Sericata Medical Maggots
Hindawi Publishing Corporation BioMed Research International Volume 2016, Article ID 8285428, 27 pages http://dx.doi.org/10.1155/2016/8285428 Research Article Next Generation Sequencing Identifies Five Major Classes of Potentially Therapeutic Enzymes Secreted by Lucilia sericata Medical Maggots Zdenjk Franta,1 Heiko Vogel,2 Rüdiger Lehmann,1 Oliver Rupp,3 Alexander Goesmann,3 and Andreas Vilcinskas1,4 1 Department of Bioresources, Fraunhofer Institute for Molecular Biology and Applied Ecology, Winchesterstraße 2, 35394 Giessen, Germany 2Department of Entomology, Max Planck Institute for Chemical Ecology, Hans-Knoll-Straße¨ 8, 07745 Jena, Germany 3Justus-Liebig-University of Giessen, Bioinformatics and System Biology, Heinrich-Buff-Ring 58, 35392 Giessen, Germany 4Justus-Liebig-University of Giessen, Institute for Insect Biotechnology, Heinrich-Buff-Ring 26-32, 35392 Giessen, Germany Correspondence should be addressed to Andreas Vilcinskas; [email protected] Received 29 January 2016; Accepted 7 March 2016 Academic Editor: Yudong Cai Copyright © 2016 Zdenekˇ Franta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Lucilia sericata larvae are used as an alternative treatment for recalcitrant and chronic wounds. Their excretions/secretions contain molecules that facilitate tissue debridement, disinfect, or accelerate wound healing and have therefore -

Role of PITRM1 in Mitochondrial Dysfunction and Neurodegeneration
biomedicines Review Role of PITRM1 in Mitochondrial Dysfunction and Neurodegeneration Dario Brunetti 1,2 , Alessia Catania 2, Carlo Viscomi 3, Michela Deleidi 4, Laurence A. Bindoff 5,6, Daniele Ghezzi 2,7,* and Massimo Zeviani 8,9,* 1 Department of Medical Biotechnology and Translational Medicine, University of Milan, 20129 Milan, Italy; [email protected] 2 Unit of Medical Genetics and Neurogenetics, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20126 Milan, Italy; [email protected] 3 Department of Biomedical Sciences, University of Padova, 35131 Padova, Italy; [email protected] 4 German Center for Neurodegenerative Diseases (DZNE), 72076 Tübingen, Germany; [email protected] 5 Neuro-SysMed, Center of Excellence for Clinical Research in Neurological Diseases, Haukeland University Hospital, N-5021 Bergen, Norway; [email protected] 6 Department of Clinical Medicine, University of Bergen, N-5021 Bergen, Norway 7 Department of Pathophysiology and Transplantation, University of Milan, 20122 Milan, Italy 8 Department of Neurosciences, University of Padova, 35128 Padova, Italy 9 Venetian Institute of Molecular Medicine, 35128 Padova, Italy * Correspondence: [email protected] (D.G.); [email protected] (M.Z.) Abstract: Mounting evidence shows a link between mitochondrial dysfunction and neurodegenera- tive disorders, including Alzheimer Disease. Increased oxidative stress, defective mitodynamics, and impaired oxidative phosphorylation leading to decreased ATP production, can determine synaptic dysfunction, apoptosis, and neurodegeneration. Furthermore, mitochondrial proteostasis and the Citation: Brunetti, D.; Catania, A.; protease-mediated quality control system, carrying out degradation of potentially toxic peptides Viscomi, C.; Deleidi, M.; Bindoff, L.A.; and misfolded or damaged proteins inside mitochondria, are emerging as potential pathogenetic Ghezzi, D.; Zeviani, M. -

Handbook of Proteolytic Enzymes Second Edition Volume 1 Aspartic and Metallo Peptidases
Handbook of Proteolytic Enzymes Second Edition Volume 1 Aspartic and Metallo Peptidases Alan J. Barrett Neil D. Rawlings J. Fred Woessner Editor biographies xxi Contributors xxiii Preface xxxi Introduction ' Abbreviations xxxvii ASPARTIC PEPTIDASES Introduction 1 Aspartic peptidases and their clans 3 2 Catalytic pathway of aspartic peptidases 12 Clan AA Family Al 3 Pepsin A 19 4 Pepsin B 28 5 Chymosin 29 6 Cathepsin E 33 7 Gastricsin 38 8 Cathepsin D 43 9 Napsin A 52 10 Renin 54 11 Mouse submandibular renin 62 12 Memapsin 1 64 13 Memapsin 2 66 14 Plasmepsins 70 15 Plasmepsin II 73 16 Tick heme-binding aspartic proteinase 76 17 Phytepsin 77 18 Nepenthesin 85 19 Saccharopepsin 87 20 Neurosporapepsin 90 21 Acrocylindropepsin 9 1 22 Aspergillopepsin I 92 23 Penicillopepsin 99 24 Endothiapepsin 104 25 Rhizopuspepsin 108 26 Mucorpepsin 11 1 27 Polyporopepsin 113 28 Candidapepsin 115 29 Candiparapsin 120 30 Canditropsin 123 31 Syncephapepsin 125 32 Barrierpepsin 126 33 Yapsin 1 128 34 Yapsin 2 132 35 Yapsin A 133 36 Pregnancy-associated glycoproteins 135 37 Pepsin F 137 38 Rhodotorulapepsin 139 39 Cladosporopepsin 140 40 Pycnoporopepsin 141 Family A2 and others 41 Human immunodeficiency virus 1 retropepsin 144 42 Human immunodeficiency virus 2 retropepsin 154 43 Simian immunodeficiency virus retropepsin 158 44 Equine infectious anemia virus retropepsin 160 45 Rous sarcoma virus retropepsin and avian myeloblastosis virus retropepsin 163 46 Human T-cell leukemia virus type I (HTLV-I) retropepsin 166 47 Bovine leukemia virus retropepsin 169 48