Meiotic DNA Double-Strand Breaks and Chromosome Asynapsis in Mice Are Monitored by Distinct HORMAD2- Independent and -Dependent Mechanisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

California Hard Core
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title California Hard Core Permalink https://escholarship.org/uc/item/0g37b09q Author Duong, Joseph Lam Publication Date 2014 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California California Hard Core By Joseph Lam Duong A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in History in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Waldo E. Martin, Chair Professor Kerwin Lee Klein Professor Linda Williams Spring 2014 Copyright 2014 by Joseph Lam Duong Abstract California Hard Core by Joseph Lam Duong Doctor of Philosophy in History University of California, Berkeley Professor Waldo E. Martin, Chair California Hard Core is a narrative history of the pornographic film industry in California from 1967 to 1978, a moment when Americans openly made, displayed, and watched sexually explicit films. Two interrelated questions animate this project: Who moved the pornographic film from the margins of society to the mainstream of American film culture? What do their stories tell us about sex and sexuality in the U.S. in the last third of the twentieth century? The earlier academic literature concentrates on pornographic film and political debates surrounding it rather than industry participants and their contexts. The popular literature, meanwhile, is composed almost entirely of book-length oral histories and autobiographies of filmmakers and models. California Hard Core helps to close the divide between these two literatures by documenting not only an eye-level view of work from behind the camera, on the set, and in the movie theater, but also the ways in which consumers received pornographic films, placing the reader in the viewing position of audience members, police officers, lawyers, judges, and anti-pornography activists. -
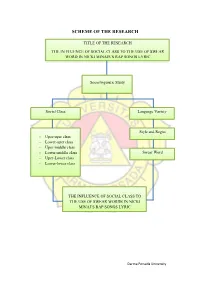
Scheme of the Research
SCHEME OF THE RESEARCH TITLE OF THE RESEARCH THE IN FLUENCE OF SOCIAL CLASS TO THE USE OF SWEAR WORD IN NICKI MINAJS’S RAP SONGS LYRIC Sociolinguistic Study Social Class Language Variety Style and Regist - Uper-uper class - Lower-uper class - Uper-middle class - Lower-middle class Swear Word - Uper-Lower class - Lower-lower class THE INFLUENCE OF SOCIAL CLASS TO THE USE OF SWEAR WORDS IN NICKI MINAJ’S RAP SONGS LYRIC Darma Persada University POSTER OF THE RESEARCH THE FLUENCE OF SOCIAL CLASS TO THE USE SWEAR WORD IN NICKI MINAJ’S RAP SONGS LYRIC Mely Arfiyanti 2015130032 DARMA PERSADA UNIVERSITY BACKGROUND FRAME WORK OF THEORIES: Sociolinguistics is the study of the relationship between language and society. How social factors can influence the Sociolinguistics Theory: way people speak. Community development; Culture, - Factors of social technology andeven religion, has caused people to form classs - Type of scoial class certian groups of social class. This form social class demands that group identities differ fromn other social Language variety classes. This need cause the effort to be different; - Style and register Included in the use of language. Each social lass seeks to be different from other sociall classes through the use of a different language. METHOD OF THE RESEARCH RESULT OF THE RESEARCH In this research, the writer use Based on the songs that the writer analyzesd, style and qualitative method to collect the data. register theory use in rap songs. They use swear The sources of the data are from words as her style asher special language that use in journals, articles, books, and internet. -

Monda Y , March 22, 2021
NATIONAL SHELLFISHERIES ASSOCIATION Program and Abstracts of the 113th Annual Meeting March 22 − 25, 2021 Global Edition @ http://shellfish21.com Follow on Social Media: #shellfish21 NSA 113th ANNUAL MEETING (virtual) National Shellfisheries Association March 22—March 25, 2021 MONDAY, MARCH 22, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound Chesapeake Bay Monterey Bay SHELLFISH ONE HEALTH: SHELLFISH AQUACULTURE EPIGENOMES & 8:30-10:30 AM CEPHALOPODS OYSTER I RESTORATION & BUSINESS & MICROBIOMES: FROM SOIL CONSERVATION ECONOMICS TO PEOPLE WORKSHOP 10:30-10:45 AM MORNING BREAK THE SEA GRANT SHELLFISH ONE HEALTH: EPIGENOMES COVID-19 RESPONSE GENERAL 10:45-1:00 PM OYSTER I RESTORATION & & MICROBIOMES: FROM SOIL TO THE NEEDS OF THE CONTRIBUTED I CONSERVATION TO PEOPLE WORKSHOP SHELLFISH INDUSTRY 1:00-1:30 PM LUNCH BREAK WITH SPONSOR & TRADESHOW PRESENTATIONS PLENARY LECTURE: Roger Mann (Virginia Institute of Marine Science, USA) (LIVE) 1:30-2:30 PM Chesapeake Bay EASTERN OYSTER SHELLFISH ONE HEALTH: EPIGENOMES 2:30-3:45 PM GENOME CONSORTIUM BLUE CRABS VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP CONSERVATION TO PEOPLE WORKSHOP BLUE CRAB GENOMICS EASTERN OYSTER & TRANSCRIPTOMICS: SHELLFISH ONE HEALTH: EPIGENOMES 3:45–5:45 PM GENOME CONSORTIUM THE PROGRAM OF THE VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP BLUE CRAB GENOME CONSERVATION TO PEOPLE WORKSHOP PROJECT TUESDAY, MARCH 23, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound -

Volume 5, Number 3, March 1937
Dear Doctor • • • • FIFTEEN Each morning I get awful pairu My head feeu like the morning after, MARCH From reading ads in subway trairu. My stummick shakes with bitter laughter. 1937 CENTS .p .-!) A HEALTHY SEX LIFE BROMO· QUININE Sneeze cures, pills, and health-Iood fads You've car-ad-sickness, that is sure How can I NEUTRALIZE these ads? HEALTH AND HYGIENE is the cure! • f Why Suffer • HEALTH AND HYGIENE I needless confusion from the chorus of false POOR FOOD and DRUG LAWS I 215 Fourth Avenue, New York City. I and extravagant advertising claims that f I clang in your ears all day ? You, too, can BY WILL MASi.OW I enclose 31. Please send HEALTH I I en joy the benefits of a sane and honest ~ AND HYGIENE for one year to magazine that gives you the truth about what you should or should not take or do I • • Name .. _. _... _..... ................................... to keep your health and native beauty. One f hundred doctors recommend HEALTH AND • Address .............................................. I HYGIENE. They write for it, they know. STORY OF INDUSTRIAL DISEASE And this is the only prescription we know I City ...................................................... I BY DR. HENRY E. SIGERIST f that's permissible to pass on to your puz f State •••••••••••••• -••••••••••••••••••••••••••••••••••••• zled friends. JOHNS HOPKINS UNIVERSITY -------- ______1 II1II( C Use the Coupon Ratings of 1937 Purely Personal In This Issue MORE AND MORE AMERICAN doctors are coming forward in aid of MARCH, 1937 Automobiles Spanish democracy in its struggle () against fascism. The unit of the VOLUME 5 NUMBER 3 DIVIDED into three price classifications under $1,000, over Medical Bureau of the American twenty· five leading models of 1937 automobiles are rated Friends of Spanish Democracy now in the current March issue of Consumers Union Reports some as "Best Buys," some as "Not Acceptable," and others as in Spain is doing notable work, hav War on Syphilis 66 ing set up a base hospital near the "Also Acceptable" in the estimated order of their merit. -

Sexual Knowledge, Attitudes and Practices of Female Undergraduate Students in Wuhan, China: the Only- Child Versus Students with Siblings
Sexual Knowledge, Attitudes and Practices of Female Undergraduate Students in Wuhan, China: The Only- Child versus Students with Siblings Shiyue Li1,2, Rucheng Chen1,2, Yue Cao1,2, Jingjing Li1,2, Dan Zuo1,2, Hong Yan1,2* 1 School of Public Health of Wuhan University, Wuhan, Hubei, P.R. of China, 2 Globe Health Center of Wuhan University, Wuhan, Hubei, P.R. of China Abstract Objectives: This study explored sexual knowledge, attitudes and practices of female only-child undergraduates and made a comparison with students with siblings. Methods: Anonymously completed questionnaires were received from 4,769 female undergraduates, recruited using randomized cluster sampling by type of university and students’ major and grade. Multivariate logistic regression was used to assess the effects of only-child on sexual knowledge, attitudes and practices among female undergraduates. Results: Of 4,769 female undergraduate students, 41.0% were only-child and 59.0% were students with siblings. Compared with students with siblings, only-child students scored higher on sex-related knowledge, were more inclined to agree with premarital sex, multiple sex partners, one-night stands, extramarital lovers and homosexuality, and were more likely to have a boyfriend and experience sexual intercourse (73.6% vs. 61.4%; 24.0% vs. 14.0%). Only-children were less likely to experience coercion at first sex and have first sexual intercourse with men not their ‘‘boyfriends’’ than children with siblings (3.3% vs. 6.4%; 20.7% vs. 28.8%). There were no significant differences on other risky sexual behaviors (e.g. multiple sex partners and inconsistent condom use) between the only-child students and students with siblings. -

Uncle Hugo's Science Fiction Bookstore Uncle Edgar's Mystery Bookstore 2864 Chicago Ave
Uncle Hugo's Science Fiction Bookstore Uncle Edgar's Mystery Bookstore 2864 Chicago Ave. S., Minneapolis, MN 55407 Newsletter #95 September — November, 2011 Hours: M-F 10 am to 8 pm RECENTLY RECEIVED AND FORTHCOMING SCIENCE FICTION Sat. 10 am to 6 pm; Sun. Noon to 5 pm Uncle Hugo's 612-824-6347 ALREADY RECEIVED Uncle Edgar's 612-824-9984 Adams, Scott Your Accomplishments Are Suspiciously Hard to Verify (Dilbert: PBO; A themed Fax 612-827-6394 collection of color and black & white comic strips showcasing the inefficiency of office life). $16.99 E-mail: [email protected] Ashley, Mike (ed) The Darker Sex (PBO; Anthology; 11 tales of the supernatural and macabre by Victorian and Website: www.UncleHugo.com Edwardian women writers).................................................$15.95 Baggott, Julianna The Ever Breath (Kids).................................................$6.99 Barclay, James Ravensoul (Legends of the Raven #4: Reissue; The Garonin are dimensional travelers seeking new Used Book Sale worlds to rape of the element of magical power. Technologically advanced in weaponry and armor, and facing only swords and magic, they are destroying everything in their path. Surely this is not a Every year our supply battle The Raven can win, even with allies both elven and dragon. But prevail they must). $16.00 (oversupply) of used books gets larger. Barnes, B/Southworth, P Runtime Error (Not Invented Here #1: PBO; Full color collection of the webcomic about a geeky We’re having a used book sale to try to software design team).....................................................$14.95 reduce our supply. Barry, Max Machine Man (PBO; When scientist Charles Neumann loses a leg in an industrial accident, it's All used books will be 20% off, an opportunity. -

ELA PRZYBYLO ASEXUAL EROTICS ABNORMATIVITIES: QUEER/GENDER/EMBODIMENT Scott Herring, Series Editor ASEXUAL EROTICS
INTIMATE READINGS OF COMPULSORY ASEXUAL SEXUALITY EROTICS ELA PRZYBYLO ASEXUAL EROTICS ABNORMATIVITIES: QUEER/GENDER/EMBODIMENT Scott Herring, Series Editor ASEXUAL EROTICS INTIMATE READINGS OF COMPULSORY SEXUALITY ELA PRZYBYLO THE OHIO STATE UNIVERSITY PRESS COLUMBUS Copyright © 2019 by The Ohio State University. All rights reserved. Library of Congress Cataloging-in-Publication Data Names: Przybylo, Ela, 1985– author. Title: Asexual erotics : intimate readings of compulsory sexuality / Ela Przybylo. Other titles: Abnormativities: queer/gender/embodiment. Description: Columbus : The Ohio State University Press, [2019] | Series: Abnormativities: queer/gender/embodiment | Includes bibliographical references and index. Identifiers: LCCN 2019009059 | ISBN 9780814214046 (cloth ; alk. paper) | ISBN 0814214045 (cloth ; alk. paper) Subjects: LCSH: Asexuality (Sexual orientation) | Sex. | Sexual attraction. | Queer theory. | Feminist theory. Classification: LCC HQ23 .P78 2019 | DDC 306.7—dc23 LC record available at https://lccn.loc.gov/2019009059 Cover design by Susan Zucker Text design by Juliet Williams Type set in Adobe Minion Pro CONTENTS List of Illustrations vi Acknowledgments vii INTRODUCTION Erotics and Asexuality: Thinking Asexuality, Unthinking Sex 1 CHAPTER 1 The Erotics of Feminist Revolution: Political Celibacies/ Asexualities in the Women’s Movement 33 CHAPTER 2 Lesbian Bed Death, Asexually: An Erotics of Failure 63 CHAPTER 3 Growing into Asexuality: The Queer Erotics of Childhood 89 CHAPTER 4 Erotics of Excess and the Aging Spinster 112 EPILOGUE Tyrannical Celibacy: The Anti-Erotics of Misogyny and White Supremacy 137 Notes 143 Bibliography 169 Index 189 INTRODUCTION Erotics and Asexuality Thinking Asexuality, Unthinking Sex IN HIS spoken word piece “A Prude’s Manifesto” (2015), poet Cameron Awk- ward-Rich announces an asexuality rarely heard or articulated. -

What If I Never Have Sex Again?
What If I Never Have Sex Again? by Susan Moon I’m afraid I’ll never have sex again. Never lie spoon to spoon with another person. I don’t feel like having sex right this minute, which is fortunate because I don’t have anybody to have it with. But I’m not sure I’ll keep on not wanting to have sex right this minute for the rest of my life. When I was younger and didn’t have a partner, I didn’t think about this. I assumed I was in between relationships. Now, at 65, I wonder if I have quietly passed beyond “in between.” Even if I did want to have sex, maybe nobody would want to have sex with me. Confidence ebbs away as skin sags in private as well as public places. I suppose you could always resort to the cover of darkness, or never taking off your nightie, but can’t fingers still feel the sag? Couples who grow old together get used to each other’s sagging in slow increments, but it’s a whole other matter to get to know somebody new when you’re already wrinkled up. Plus, I’m not as bendable as I used to be. I used to like sex a lot if I liked the person, but when I didn’t have it, I didn’t miss it much. Sometimes I missed the person. Saying I miss sex is like saying I miss wearing my hiking boots, when what I miss is standing at Paiute Pass watching the cloud shadows run across the lake below. -

UNDERSTANDING the PURPOSE and POWER of MEN a Book for Men and the Women Who Love Them
Unless otherwise indicated, all Scripture quotations are from the Holy Bible, New International Version®, NIV®, © 1973, 1978, 1984 by the International Bible Society. Used by permission of Zondervan. All rights reserved. All Scripture quotations marked (NKJV) are from the New King James Version, © 1979, 1980, 1982, 1984 by Thomas Nelson, Inc. Used by permission. All rights reserved. Scripture quotations marked (KJV) are from the King James Version of the Holy Bible. Scripture quotations marked (NASB) are from the New American Standard Bible ® , NASB ®, © 1960, 1962, 1963, 1968, 1971, 1972, 1973, 1975, 1977, 1988 by The Lockman Foundation. Used by permission. (www.Lockman.org) UNDERSTANDING THE PURPOSE AND POWER OF MEN a book for men and the women who love them Dr. Myles Munroe Bahamas Faith Ministries International P.O. Box N9583 Nassau, Bahamas e-mail: [email protected] websites: www.bfmmm.com; www.bfmi.tv; www.mylesmunroe.tv ISBN-13: 978-0-88368-725-3 ISBN-10: 0-88368-725-9 Printed in the United States of America © 2001 by Dr. Myles Munroe Whitaker House 1030 Hunt Valley Circle New Kensington, PA 15068 www.whitakerhouse.com Library of Congress Cataloging-in-Publication Data Munroe, Myles. Understanding the purpose and power of men / by Myles Munroe. p. cm. ISBN 0-88368-725-9 (pbk. : alk. paper) 1. Men (Christian theology) 2. Sex role—Religious aspects—Christianity. I. Title. BT703.5 .M86 2002 261.8'3431—dc21 2001007727 No part of this book may be reproduced or transmitted in any form or by any means, electronic or mechanical—including photocopying, recording, or by any information storage and retrieval system—without permission in writing from the publisher. -

Ms Week VALLEY PIONEER of AT
Ms Week VALLEY PIONEER Of AT TWENTY-FOURTH YEAR. DAUBHTERJUESDAY By Arthur Britban RIGBY. JEFFERSON COUNTY, IDAHO, THURSDAY, SEPTEMBER 16, 1926. NUMBER FORTY-ONE Grim Reaper Closes GOETHE READ THEM. France-Belgium Senator W. E. Borah Active Life Of Ed 0 OCTOPUS' CHILDREN. STATE Ai COUNT! Beneficiaries in Will DISTRICT FAIR AT Rigby Visitor Sat. mund Ellsworth; Was 11 IE OF WEALTH. SUPREME COURT Grandson of Brigham ^ TWIN FALLS. Idaho, Sept. 14.— Senator Wm. E. Borah was the Young. PI1AL PUNISHMENT? TAXES ARE $11,000 Frank H. Buhl, foremost among the gueat of Mr. and Mrs. J. W. Hart builders of the Twin Falls country OLAGKFOOTOPENS here Saturday night, the aeuator re ;AH! JUDGE PROBABLY and president of the Twin Falls Land turning that night from the Bechlar Jefferson county lost one of ita aer company prior to hia death Meadows, southwestern corner of Yel moat genteel old gentlemen Tueaday ^the said the beat way to Ger- in li»18, bequeathed one million dol at noon when Edmund Ellsworth Sr. ,iie territory taken from Poland lowstone park, and proposed l LESS THAI IN 1925 lars to France and another million TUESDAY. SEPT. 2 voir site for the Fremont-Madison of Lewlavllle passed to his reward. :io send German plays and play- to Belgium, it was disclosed in a re- "Grandpa", as he has been known to inspire respect for the Ger- canals. !0 Reduction In State and special dispatch to the Chicago Senator Borah joined the party oi three generations, closed an enviable aud language, Tribune, a copy of which followa: Over $16,000 In Prem career in his passing and his mem fiat would Goethe think of theae Superintendent Albright at Bechlar Geo. -

Thin on the Ground
Advances in Human Biology t Matt Cartmill and Kaye Brown, Series Editors THIN ON THE GROUND Neandertal Biology, Archeology, and Ecology Steven E. Churchill JWST461-c01 JWST461-Churchill Printer: Yet to Come June 11, 2014 6:52 Trim: 254mm×178mm JWST461-fm JWST461-Churchill Printer: Yet to Come July 7, 2014 7:14 Trim: 254mm×178mm Thin on the Ground JWST461-fm JWST461-Churchill Printer: Yet to Come July 7, 2014 7:14 Trim: 254mm×178mm Advances in Human Biology Series Editors: Matt Cartmill Kaye Brown Boston University Titles in this Series Thin on the Ground: Neandertal Biology, Archeology, and Ecology by Steven E. Churchill JWST461-fm JWST461-Churchill Printer: Yet to Come July 7, 2014 7:14 Trim: 254mm×178mm THIN ON THE GROUND Neandertal Biology, Archeology, and Ecology STEVEN EMILIO CHURCHILL Department of Evolutionary Anthropology Duke University USA SERIES EDITORS: MATT CARTMILL AND KAYE BROWN JWST461-fm JWST461-Churchill Printer: Yet to Come July 7, 2014 7:14 Trim: 254mm×178mm This edition first published 2014 © 2014 by John Wiley & Sons, Inc. Editorial offices: 1606 Golden Aspen Drive, Suites 103 and 104, Ames, Iowa 50010, USA The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 9600 Garsington Road, Oxford, OX4 2DQ, UK For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell. Authorization to photocopy items for internal or personal use, or the internal or personal use of specific clients, is granted by Blackwell Publishing, provided that the base fee ispaid directly to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923. -

Artist Song Album Blue Collar Down to the Line Four Wheel Drive
Artist Song Album (BTO) Bachman-Turner Overdrive Blue Collar Best Of BTO (BTO) Bachman-Turner Overdrive Down To The Line Best Of BTO (BTO) Bachman-Turner Overdrive Four Wheel Drive Best Of BTO (BTO) Bachman-Turner Overdrive Free Wheelin' Best Of BTO (BTO) Bachman-Turner Overdrive Gimme Your Money Please Best Of BTO (BTO) Bachman-Turner Overdrive Hey You Best Of BTO (BTO) Bachman-Turner Overdrive Let It Ride Best Of BTO (BTO) Bachman-Turner Overdrive Lookin' Out For #1 Best Of BTO (BTO) Bachman-Turner Overdrive Roll On Down The Highway Best Of BTO (BTO) Bachman-Turner Overdrive Take It Like A Man Best Of BTO (BTO) Bachman-Turner Overdrive Takin' Care Of Business Best Of BTO (BTO) Bachman-Turner Overdrive You Ain't Seen Nothing Yet Best Of BTO (BTO) Bachman-Turner Overdrive Takin' Care Of Business Hits of 1974 (BTO) Bachman-Turner Overdrive You Ain't Seen Nothin' Yet Hits of 1974 (ELO) Electric Light Orchestra Can't Get It Out Of My Head Greatest Hits of ELO (ELO) Electric Light Orchestra Evil Woman Greatest Hits of ELO (ELO) Electric Light Orchestra Livin' Thing Greatest Hits of ELO (ELO) Electric Light Orchestra Ma-Ma-Ma Belle Greatest Hits of ELO (ELO) Electric Light Orchestra Mr. Blue Sky Greatest Hits of ELO (ELO) Electric Light Orchestra Rockaria Greatest Hits of ELO (ELO) Electric Light Orchestra Showdown Greatest Hits of ELO (ELO) Electric Light Orchestra Strange Magic Greatest Hits of ELO (ELO) Electric Light Orchestra Sweet Talkin' Woman Greatest Hits of ELO (ELO) Electric Light Orchestra Telephone Line Greatest Hits of ELO (ELO) Electric Light Orchestra Turn To Stone Greatest Hits of ELO (ELO) Electric Light Orchestra Can't Get It Out Of My Head Greatest Hits of ELO (ELO) Electric Light Orchestra Evil Woman Greatest Hits of ELO (ELO) Electric Light Orchestra Livin' Thing Greatest Hits of ELO (ELO) Electric Light Orchestra Ma-Ma-Ma Belle Greatest Hits of ELO (ELO) Electric Light Orchestra Mr.